KEY CONCEPTS
•
A number of parameters must be taken into consideration in the selection of boundary lubricity additives for use in MWFs.
•
Boundary lubricity additives with specific functionalities can display multiple functions in MWFs.
•
The wide variety of boundary lubricity additives means that multiple ones can be used in a specific MWF to meet performance requirements, particularly in machining nonferrous alloys, such as aluminum.
•
Newer boundary lubricity additives are in use that combine the benefits of ester-based components with EP agents.
The challenges facing metalworking fluid (MWF) formulators usually focus on such issues as minimizing microbial contamination, eliminating foam and having products that can operate under harsh operating conditions, particularly with high levels of water hardness. But MWFs need to contain the proper types of lubricity components to fulfill their main function of reducing friction and wear.
Components used in MWFs to assist with this function are known as boundary lubricity additives. They typically contain a polar group that interacts with the metal surface and a tail functionality that is compatible with the fluid carrier, whether it be mineral oil or water.
Boundary lubricity additives function by adsorbing on the metal surface to produce a film that will reduce contact at the interface where a tool interacts with a workpiece. Figure 1 shows how the polar head of the boundary lubricity additive is positioned on the metal surface with the tail in the MWF. The main types of boundary lubricity additives are esters and fatty oils (such as canola oil).
At the point where the gap between the tool and workpiece becomes very small and the metalworking operation enters the boundary lubrication regime, a special type of boundary lubricity additive is needed to prevent galling and welding between the two metal surfaces that are in close proximity. This additive class is known as extreme pressure (EP) agents, and the three most common types (based on chlorine, phosphorus and sulfur) function through an activation mechanism by reacting with the metal surface at elevated temperatures to form metal chlorides, phosphates and sulfides, respectively.
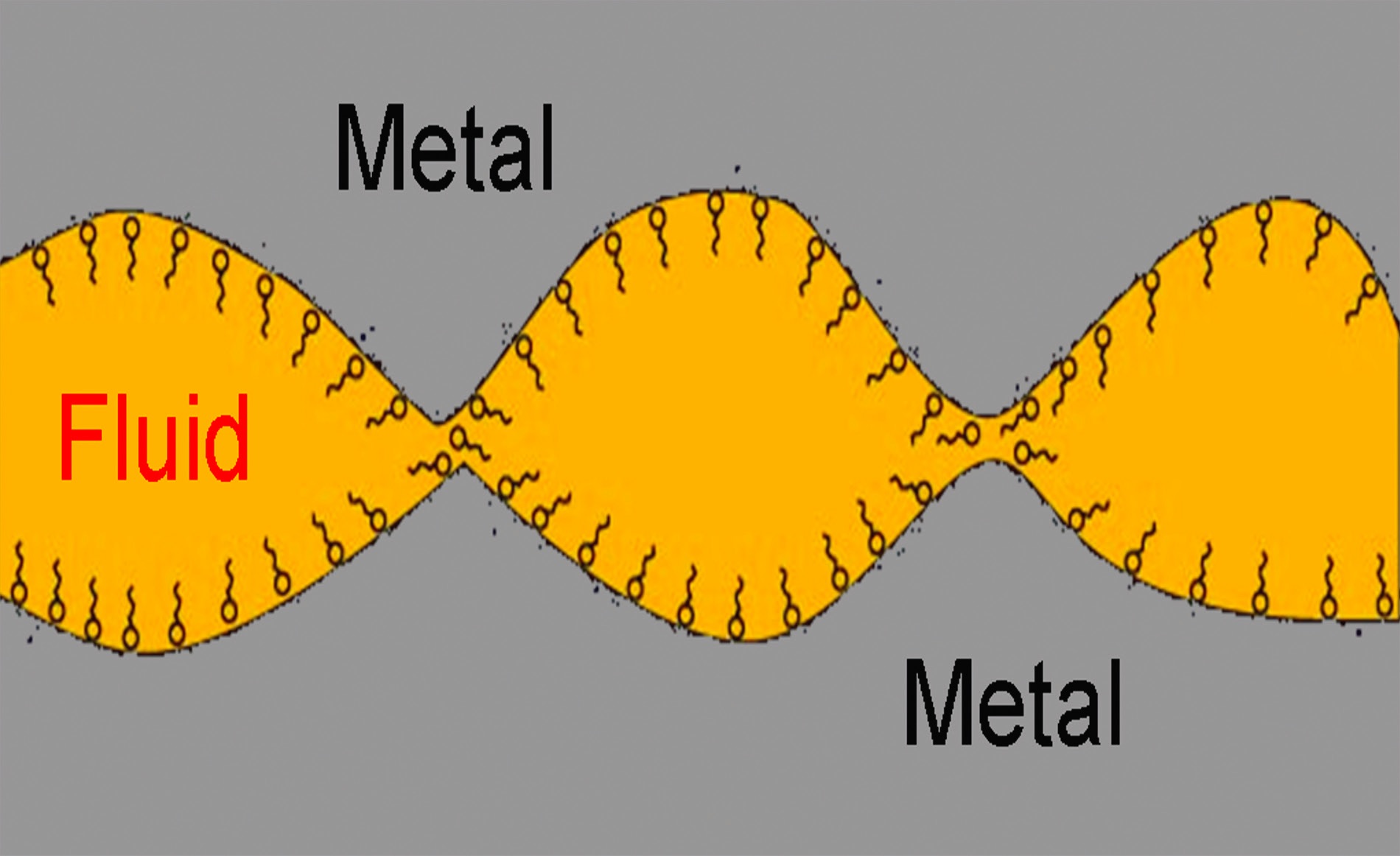 Figure 1. The polar heads (represented by the circles) of boundary lubricity additives adsorb on the metal surface while their squiggly tails, compatible with the base oil used, are positioned in the MWF. Figure courtesy of STLE.
Figure 1. The polar heads (represented by the circles) of boundary lubricity additives adsorb on the metal surface while their squiggly tails, compatible with the base oil used, are positioned in the MWF. Figure courtesy of STLE.
The purpose of this article is to provide an update on the types of boundary lubricity additives that are available to deal with multiple challenges facing the MWF formulator.
Input on the issue was obtained from industry experts who have perspectives from the additive, independent consultant and formulator standpoints.
The following individuals were contacted.
1.
Marc Chan, Clariant
2.
Stephanie Cole, Clariant
3.
Richard Butler, DuBois Chemicals
4.
Scott Cheng, Ingevity
5.
Gary Andrews, Innovative Fluid Design
6.
Ron Lemke, Italmatch
7.
Ben Faber, The Lubrizol Corp.
8.
Yew-Chong Lai, Oleon.
Key selection parameters
Yew-Chong Lai, research engineer for Oleon in Port Klang, Malaysia, lists four parameters that must be taken into consideration in selecting boundary lubricity additives. He says, “Factors that must be evaluated include good performance on different metals, good compatibility with different base oils, low corrosiveness to yellow metals and sustainability and environmental impact.”
Further details on each issue are listed below.
• Good performance on different metals. Boundary lubricity additives that contain sulfur, phosphorus and chlorine rely on the activation temperature and pressure conditions to form sufficient films to protect the metal surfaces. However, due to the activity of the specific EP additive and the hardness of the metal undergoing machining, in some cases, the activation temperature might not be achieved during machining. This reduces the protection that EP additives can provide to different metals. Formulators must work with the available EP additives to ensure protection of different metal alloys during challenging machining operations.
• Good compatibility with different base oils. The shift in base oil use from Group I to Group II and III might create incompatibility/solubility issues between additives used in MWF formulations and base oil. The lower aromatic content present in Group II and III base oils makes them inferior solvents when compared to Group I. Proper selection of boundary lubricity additives is needed to ensure compatibility with a wide range of base oils to minimize the concern about phase separation or precipitation that could lead to poor MWF performance.
• Low corrosiveness to yellow metals. Yellow metals such as copper and bronze are susceptible to corrosion when EP additives are used in general and sulfurized additives are used in particular. Instead of protecting the metal, the boundary lubricity additive might stain or corrode. This might require the use of additional yellow metal deactivators to provide protection against yellow metal corrosion, introducing additional cost and complicating a formulation where more additives might compete for space on the metal surface.
• Sustainability and low environmental impact. There are no requirements yet for MWF formulations to comply with Ecolabel (EU) and BioPreferred (U.S.) programs, but interest is growing in having acceptable biodegradability for disposal purposes. Development of safe and low eco-toxicity boundary lubricity additives could reduce disposal costs as these additives might replace more hazardous substances such as chlorinated paraffins.
Ben Faber, North American product manager – metalworking for The Lubrizol Corp. in Wickliffe, Ohio, says, “Two key parameters must be considered when selecting boundary lubricity additives. Chemical stability must be ensured, which means the additive must be soluble in the diluent used (water or oil), must be oxidatively stable at higher temperatures, must be compatible with amines and other additives required for MWF formulations and must be hydrolytically stable in the case of water-based MWFs. In other words, the boundary lubricity additive must survive robustly in the MWF long enough to provide performance. Secondly, the additive must provide suitable lubricity performance for the application and metallurgy of the operation.”
Faber also points out that additional important items to consider in the selection process include hazard statements on the safety data sheet and global chemical inventory registrations.
STLE member Stephanie Cole, formulation chemist for Clariant in Mt. Holly, N.C., indicates that a formulator must first understand the target application of the product. She says, “One of the highest priorities is to have a strong understanding of the temperature, pressure, tool speed, feed rates, metal substrate, etc., that are involved in the specific machining operation. In moving to the formulation bench, the end-goal when selecting a boundary lubricity additive is to attain formulation stability. It is crucial that all ingredients maintain a synergy in the formulation to ensure that the delivered product is stable during transportation and storage.”
Oxidative stability is another important criterion for a boundary lubricity additive. Cole explains, “The additive must withstand elevated temperatures and not form a sticky residue that can lead to machine malfunction and product failure.”
Two other considerations pointed out by Cole are cloud point and foam. She says, “The boundary lubricity additive functions by popping out of the diluted MWF at a certain temperature, allowing for proper lubrication during the critical moment at the tooling-substrate interface. Product characteristics such as cloud point help provide some insight to an additive’s performance. The boundary lubricity additive’s contribution to foam also is important to understand.”
STLE member Scott Cheng, global technical lead – lubricants for Ingevity in North Charleston, S.C., offers a list of requirements that formulators must consider in selecting a boundary lubricity additive. He says, “Boundary lubricity additives must be selected based on the solubility, and/or ease of incorporation, in the MWF base formulation. They must not harm the metal surface, decompose or breakdown under operational conditions. Boundary lubricity additives also must be compatible with “downstream” processes such as cleaning, surface treatments, coatings and heat treating. Environmental health & safety (EH&S) standards and registrations for markets where the MWF will be offered also must be taken into consideration.”
STLE member Ron Lemke, business development and OEM manager for Italmatch in Cleveland, Ohio, considers the primary factor in selecting a boundary lubricity additive to be the metal(s) or materials being machined. He adds, “Secondary considerations would be the machining operations and other functions that a formulator would like to have from the additive, including co-emulsification, bioresistance, corrosion protection and possibly EP functionality.”
Positive and negative characteristics
Cole indicates that there are positive aspects to using boundary lubricity additives that do not exhibit EP characteristics. She says, “Boundary lubricity additives tend to outperform in the aspects of corrosion issues, product handling, safety labels and environmental impact. They also tend to be multifunctional across a variety of formulations as they provide lubrication, some emulsification properties and contribute to corrosion protection.”
Cole emphasizes that in some applications, formulators cannot rely just on a single additive and need to formulate several boundary lubricity additives, including an EP agent to efficiently lower the coefficient of friction in a specific application.
Cheng says, “Multiple chemical options that cover a wide range of temperatures and other operating conditions represent a positive characteristic for boundary lubricity additives. Some of the additives in this category display very effective performance at low treat rates.”
Figure 2 illustrates that boundary lubricity additives are effective over a wide temperature range. Fatty ester-based additives work well at lower temperatures while EP agents contribute significantly at higher temperatures. Tall oil fatty esters appear to bridge a temperature gap between fatty oils and esters and EP agents.
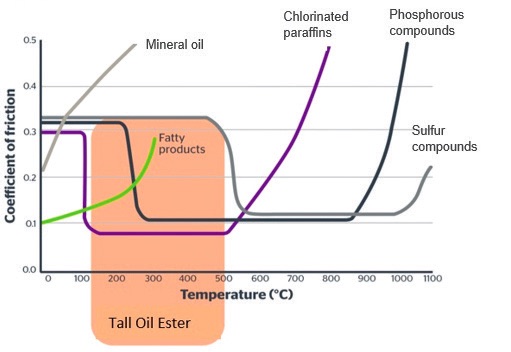 Figure 2. The performance of several types of boundary lubricity additives over a temperature range from 0 to 1,100 C is shown. Tail oil esters are effective over a broader temperature range and act as a bridge between fatty oils and esters and EP agents. Figure courtesy of Ingevity.
Figure 2. The performance of several types of boundary lubricity additives over a temperature range from 0 to 1,100 C is shown. Tail oil esters are effective over a broader temperature range and act as a bridge between fatty oils and esters and EP agents. Figure courtesy of Ingevity.
On the negative side, Cheng expressed concern specifically about phosphorus-based boundary lubricity additives. He says, “At least in the U.S., phosphorus-containing additives have come under increasing scrutiny regarding MWF disposal and other situations where environmental contamination is a possibility.”
Lemke indicates that boundary lubricity additives with specific functionalities can display multiple functions, which is desirable for MWF formulators. He says, “Polymerized fatty acids are very effective on aluminum alloys and other soft alloys of copper and magnesium. Besides exhibiting good lubricity, they also aid in co-emulsification due to displaying low hydrophilic-lipophilic balance values that are a nice offset to the high hydrophilic-lipophilic balance of other emulsifiers such as sodium petroleum sulfonates. A second additive type that shows versatility is phosphate esters that provide excellent boundary lubrication but also will aid in corrosion inhibition with yellow metals. A final example is an inactive sulfurized ester that combines the good boundary lubrication of the fatty acid of the ester with sulfur functionalities that provide good polarity for bonding to the metal substrate. The net result is a great boundary lubricity additive.”
Faber focused on the different types of esters available for use as the most common boundary lubricity additive in MWFs. He says, “Esters contain a polar functional group that has a high affinity to a metal surface, is relatively chemically stable and is readily available in nature or easy to manufacture synthetically. This class of additives can be considered simple in shape as shown by the examples of triglycerides or methyl esters. These additives are low in cost, globally available but can struggle at higher temperatures due to their unsaturation, which limits application life compared to esters synthesized from synthetic ingredients.”
Faber states that complex polymeric esters will generally perform better than those esters simple in shape. He says, “Polymeric esters have more anchor points to a metal surface than simple esters, which increases film strength, plus they have a higher viscosity index and maintain higher viscosity during metalworking operations. This ester type is generally more chemically stable because their large complex and often branched shapes generally increase steric hindrance, improving aminic and hydrolytic stability of the ester functional groups.”
Lai believes that boundary lubricity additives based on sulfur are a big concern due to their incompatibility with yellow metals. He says, “Sulfurized EP additives containing active sulfur might chemically attack the surface of softer yellow metals, causing corrosive damage.”
Performance on hard-to-machine alloys
Faber indicates that the mechanism ester-based boundary lubricity additives use to function is different than for EP additives. He says, “Esters adsorb to the metal surface due to electrical attraction, which provides vastly more versatility compared to the chemically reactive mechanism that EP additives use in reacting with the metal surface to form a new layer. The polar heads of esters are attracted to basically all metal surfaces, while currently used EP additives are optimized to work best on iron-containing metals.”

The value of using esters is pronounced in working with non-ferrous metals that are growing in popularity in a variety of industries due to their lightweighting properties. Faber says, “Esters have been successfully used to cut and form these metals. Formulators have developed specially designed fluids for aluminum, titanium, copper and other non-ferrous metals that often rely exclusively on esters or synergistic combinations of esters and EP additives for their lubricity performance.”
The performance of a complex polymeric ester is shown in Figure 3. A tapping torque study was used to evaluate a synthetic MWF base formula containing an ethylene oxide/propylene oxide (EO/PO) block copolymer versus the same base formula that is prepared with a lower amount of a water extendable polymeric ester. The study involved tapping 6061 aluminum and G5 titanium with fluid dilutions at 5% and 10%, respectively. Higher relative efficiency values were realized with the polymeric ester versus the EO/PO block copolymer on both non-ferrous metal alloys.
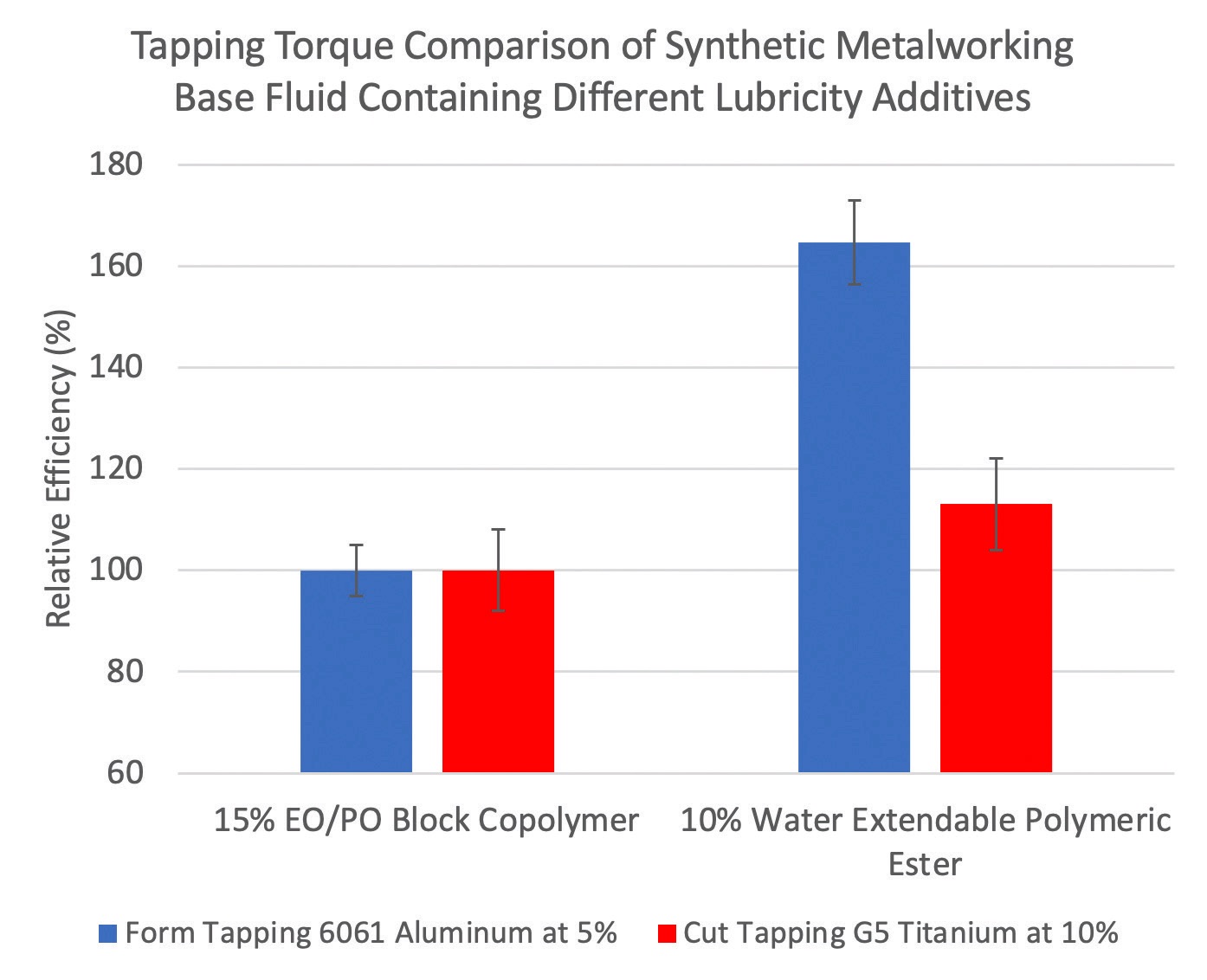 Figure 3. Tapping torque comparison between a synthetic MWF base formula containing an ethylene oxide/propylene oxide (EO/PO) block copolymer (left) versus the same base fluid with a lower amount of a water extendable polymeric ester (right). Tapping was done on 6061 aluminum with 5% concentration (blue) and on G5 titanium with 10% concentration (red). In both instances, the polymeric ester provided higher relative efficiency (better lubricity) than the EO/PO polymer. Figure courtesy of The Lubrizol Corp.
Figure 3. Tapping torque comparison between a synthetic MWF base formula containing an ethylene oxide/propylene oxide (EO/PO) block copolymer (left) versus the same base fluid with a lower amount of a water extendable polymeric ester (right). Tapping was done on 6061 aluminum with 5% concentration (blue) and on G5 titanium with 10% concentration (red). In both instances, the polymeric ester provided higher relative efficiency (better lubricity) than the EO/PO polymer. Figure courtesy of The Lubrizol Corp.
Lemke feels that boundary lubricity additives will be effective on non-ferrous alloys. He says, “Boundary lubrication is the main mode of lubrication with softer alloys, such as aluminum, magnesium and most copper alloys.
Cole points out that one reason for the effectiveness of non-EP, boundary lubricity additives in machining aluminum is operations do not typically involve higher temperatures. She says, “The boundary lubricant additive acts as a barrier between the cutting or forming tool piece and the metal substrate to change the shape of the workpiece without creating structural defects. This additive assists in the lower temperature phases of the cutting and forming process when machining difficult-to-cut materials such as steel, titanium and Inconel.”
Cole emphasizes that an EP additive based on chlorine, sulfur and phosphorus also might be required for applications using difficult-to-machine alloys. She says, “Activation of these EP additives at higher temperatures might be needed to ensure proper cooling and increased tool life.”
The benefit of employing more than one boundary lubricity additive is shown in Figure 4, which documents a microtap study on 1018 steel and 7075 aluminum. Four fluids were used with the control being a water-based synthetic MWF formulation. A complex ester and a polyalkylene glycol (PAG) were each added to the formulation separately and in combination.
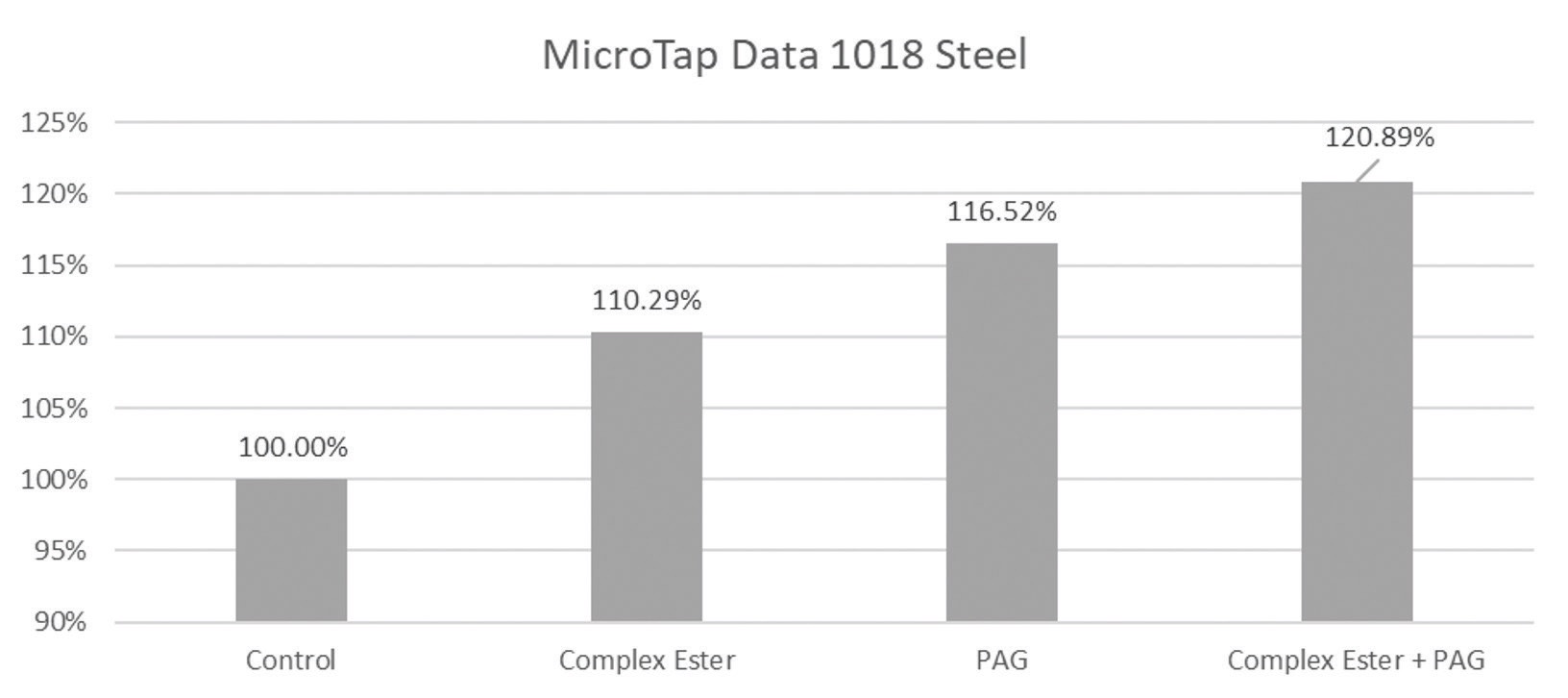
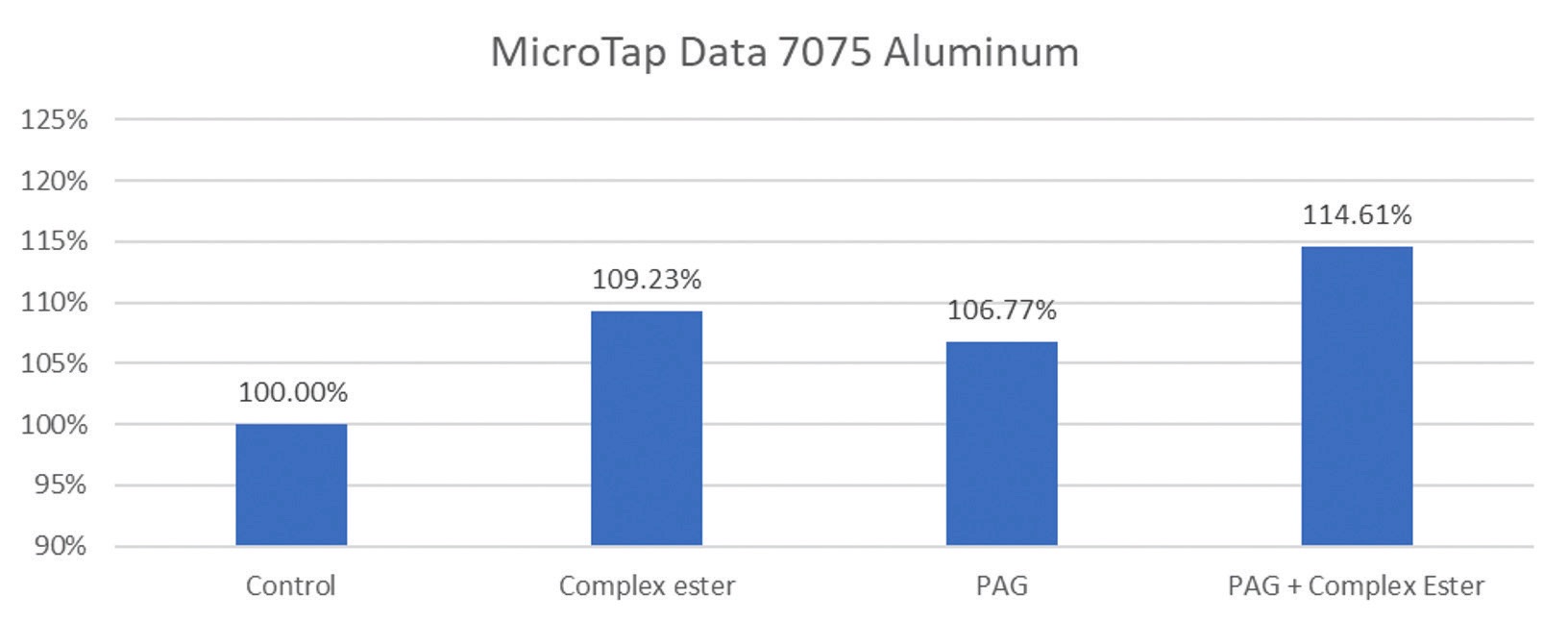 Figure 4. Microtap data shows how the addition of specific additives to a synthetic MWF affects performance on 1018 steel (top graph) and 7075 aluminum (bottom graph). A significant increase in lubricity performance on both metal alloys was found when a complex ester and a polyalkylene glycol (PAG) were added to the control (see furthest chart on the right of each graph). Figure courtesy of Clariant.
Figure 4. Microtap data shows how the addition of specific additives to a synthetic MWF affects performance on 1018 steel (top graph) and 7075 aluminum (bottom graph). A significant increase in lubricity performance on both metal alloys was found when a complex ester and a polyalkylene glycol (PAG) were added to the control (see furthest chart on the right of each graph). Figure courtesy of Clariant.
For the 1018 steel test, the fluids were diluted to 7% in water, and the RPM was set to 500. With the 7075 aluminum test, the fluids were diluted to 10% in water, and the RPM was set to 1000.
A 10% increase in lubrication (relative efficiency) was found when adding the complex ester to the synthetic MWF formulation. Combining the complex ester with the PAG produced even better relative efficiency values.
Replacing chlorinated paraffin
Regulatory concerns have led to limiting the use of chlorinated paraffins. Non-chlorine containing boundary lubricity additives have been under evaluation as alternatives.
Lai stated that a sulfur-based boundary lubricity additive based on a thiolated polyglycerol ester has displayed promise as a substitute for chlorinated paraffins in mild extreme pressure machining. He says, “The thiolated ester exhibits the film forming capability of a polyglycerol ester and the ability of the thiol group to form metal-sulfide film under extreme pressure conditions.”
A field trial using a CNC-lathe machine in a turning operation was conducted on straight oils formulated with the thiolated polyglycerol ester and a commercial sulfurized additive that contains active sulfur. Both additives were used at a 5% treat rate in naphthenic oil.
The force required for the workpiece to move in the “x” and “z” directions was measured, and the results are shown in Figure 5 on the tool steel alloy SKD11 and the medium carbon steel AISI 1045. Lai says, “The results show that the thiolated ester displays similar performance to the sulfurized additive on SKD11 and superior performance on ASI 1045. This boundary lubricity additive seems to be versatile on alloys with different hardness ratings. We believe the thiolated ester provides an efficient lubrication film on newly machined surfaces and sustained lubricity to the cutting tool.”
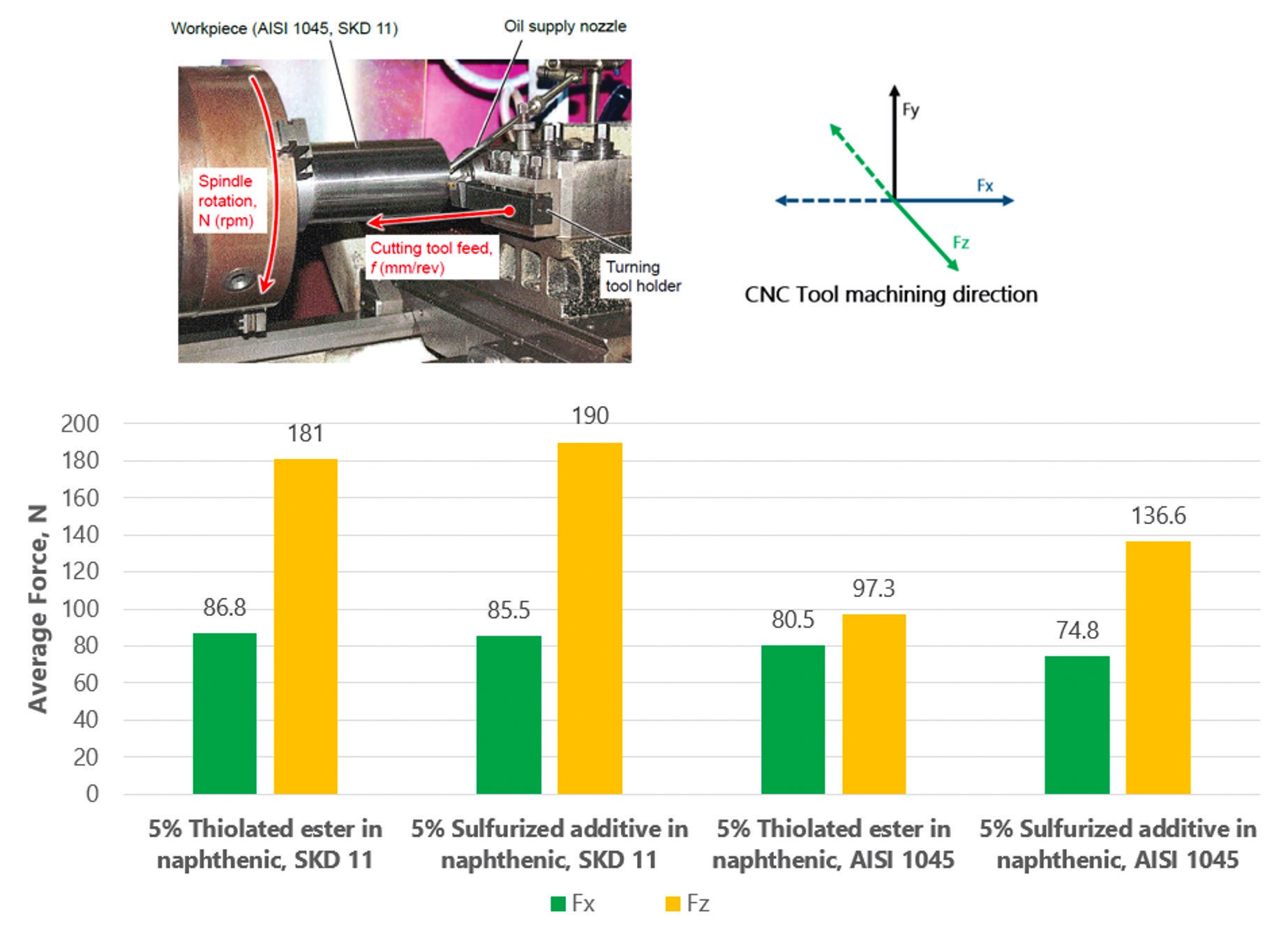 Figure 5. The results from a field trial that involved using a CNC-lathe machine (see top left hand image) and the data presented in two graphs are shown. The force required for the workpiece to move in the “x” (green bar charts) and “z” (yellow bar charts) directions is shown in applications using the tool steel alloy, SKD11, and the medium carbon steel alloy, AISI 1045. In both cases, the thiolated ester used at a 5% treat rate demonstrated comparable performance to a commercial sulfurized additive used at the same treat rate on SKD11 and superior performance on AISI 1045. Figure courtesy of Oleon.
Figure 5. The results from a field trial that involved using a CNC-lathe machine (see top left hand image) and the data presented in two graphs are shown. The force required for the workpiece to move in the “x” (green bar charts) and “z” (yellow bar charts) directions is shown in applications using the tool steel alloy, SKD11, and the medium carbon steel alloy, AISI 1045. In both cases, the thiolated ester used at a 5% treat rate demonstrated comparable performance to a commercial sulfurized additive used at the same treat rate on SKD11 and superior performance on AISI 1045. Figure courtesy of Oleon.
Tooling wear data generated during the study shows that the thiolated ester produces lower flank wear than the sulfurized additive on both alloys (SKD11 and AISI 1045) as shown in Figure 6. Lai says, “The thiolated ester has the potential to prolong tool life.”
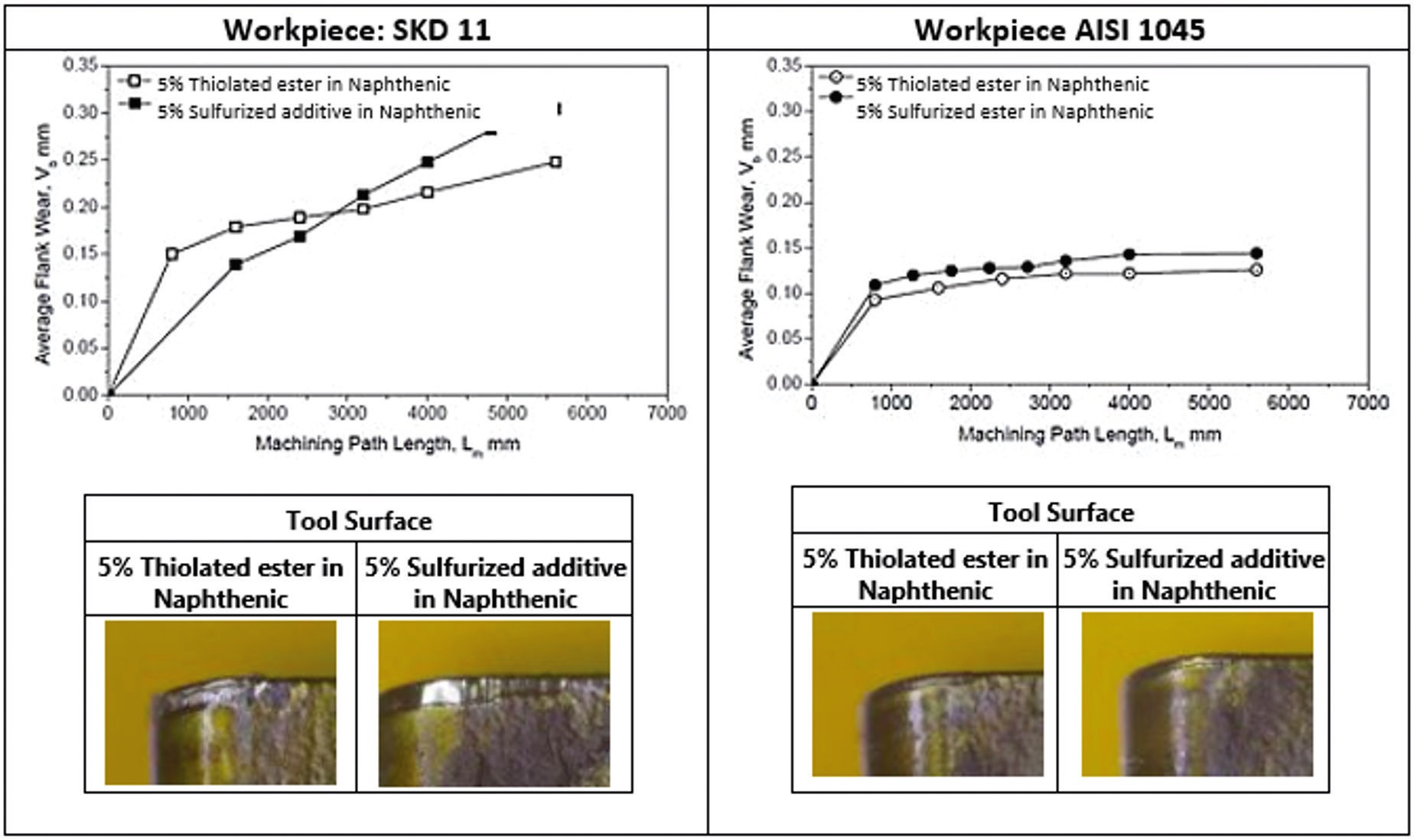 Figure 6. Tooling wear data generated in the CNC-lathe machine study is shown in the two graphs on SKD11 and AISI 1045. Images of the actual tool surfaces used in machining both alloys is shown below the graphs. The thiolated ester exhibits lower flank wear than the commercial sulfurized additive on both alloys. Figure courtesy of Oleon.
Figure 6. Tooling wear data generated in the CNC-lathe machine study is shown in the two graphs on SKD11 and AISI 1045. Images of the actual tool surfaces used in machining both alloys is shown below the graphs. The thiolated ester exhibits lower flank wear than the commercial sulfurized additive on both alloys. Figure courtesy of Oleon.
Another benefit is the thiolated ester contains no active sulfur and generates a 1b copper strip in the copper corrosion test (ASTM D130). In contrast, the sulfurized additive used in the turning study has a significant active sulfur content and produces a 4b copper strip in the same test.
Cheng feels that no boundary lubricity additives are currently available that exhibit the performance and ease of use seen with chlorinated paraffins. He says, “Non-EP-based boundary lubricity additives can assist with MWF performance by providing lubrication in situations where the activation energy is below the level needed for more acceptable EP additives based on sulfur and phosphorus. Polymeric high molecular weight additives can provide assistance under certain conditions by forming protective thin films at the tool-workpiece interface.”
A combination of base oil, a complex ester and a phosphate ester might, in some cases, assist with filling the gap left by not having chlorinated paraffins available to the formulator, according to Cole. She says, “In theory, this combination would provide sufficient lubrication at the lower temperature range of where chlorinated paraffins can operate. But chlorinated paraffins are difficult to replace because their temperature operating range is still quite broad, which enables them to provide lubrication at the immediate point of cut and remain active through the cutting process.”
Faber admits that replacement of chlorinated paraffins is difficult in the most extreme operations, particularly involving difficult-to-machine metals such as stainless steel and superalloys and in difficult applications such as deep drawing and broaching. He says, “Esters and synergistic combinations of esters and EP additives can replace chlorinated paraffins in many medium- and light-duty applications. Some well-known and utilized synergistic combinations include the use of polymer esters and sulfurized olefins and the use of polymer esters and phosphate esters. Chlorine-free fluids based on these formulation approaches are gaining popularity globally and growing in market share.”
Formulator perspective
Input was requested from two MWF formulators about the challenges faced in selecting boundary lubricity additives for specific applications. STLE member Gary Andrews, principal at Innovative Fluid Design in Shorewood, Ill., says, “Formulators depend on their suppliers to provide performance data to show how a specific boundary lubricity additive rates versus industry standards. There is insufficient time to screen all available boundary lubricity additives for a specific application. Plus, the cost involved is prohibitive. STLE member Richard Butler, R&D manager of metalworking fluids for DuBois Chemicals in Bedford Park, Ill., says, “Boundary lubricity additives are particularly important in aerospace and medical implant applications. Two key properties that are required for any additive are to be non-corrosive and non-staining.”
Butler revealed that there are a limited number of boundary lubricity additives that are effective on non-ferrous alloys. He says, “Chlorinated organic derivatives work well on both ferrous and aluminum alloys. Brass and bronze alloys can be handled through free machining and do not require boundary lubricity additives. Another option for formulators is to use calcium-based passive EP additives that work well with all alloys (ferrous and non-ferrous).”
Andrews states that it is now easier to identify boundary lubricity additives because suppliers are using industry pertinent tests such as the microtap to evaluate new candidates on a variety of metal substrates. He says, “Microtap and other laboratory test data can provide insight on the performance of a specific boundary lubricity additive, but they will not replace actual field trials, which are more valuable. Gaining access to actual machine tools provides an environment where a variety of different substrates and applications can usually be tested.”
Performance issues faced by formulators can be overcome if suppliers are willing to use them in better model formulations, according to Andrews. He says, “Suppliers often provide test data in simple matrices such as a naphthenic base oil or water, which do not indicate how a boundary lubricant additive might affect a formulation’s concentrate or emulsion stability. There is need for better guide formulations to assist with letting formulators know how a specific new additive might fit in with their approach in constructing MWFs.”
Andrews believes that high performing boundary lubricity additives are now commercially available. He adds, “The desire to get better tool life or better surface finishes in difficult machining applications will always be evident. This means that MWF companies will remain interested in new additive technology that can surpass what is available today.”
One concern that Butler raised is the packaging used by MWF manufacturers in shipping specific types of lubricants containing EP additives. He says, “Many corrosive sulfur and chlorinated additives can react in the storage containers of finished MWFs. This is particularly true in hotter climates where lubricant drums are frequently stored outdoors in direct sunlight.”
On the subject of formulating away from chlorinated paraffins, both individuals believe that alternatives exist, except in specific cases. Butler says, “Very few metal-removal applications absolutely require chlorinated paraffins any longer. Multiple sulfur, phosphorus and calcium additives can effectively replace chlorinated organics. A very few metalforming operations still require chlorinated paraffins.”
Andrews says, “In applications and metallurgies where chlorinated EP additives excel, no existing non-chlorine-based boundary lubricity additives exist that can match their performance and certainly not at an equivalent cost. Some boundary lubricity additives can bridge the gap and, in combination with EP additives (such as phosphate esters and sulfurized components), can be an extremely valuable component of a formulation.”
Bioresistance is another factor that formulators are taking into consideration in selecting additives. Andrews says, “Boundary lubricity additives do not appear to provide any positive bioresistance benefit. In fact, ester-based additives can negatively impact a fluid’s resistance to bacterial contamination. The best way to determine bioresistance is to run challenge tests on finished fluids that have identical compositions, with the exception of the additive under evaluation.”
Butler feels that some EP additives are vulnerable to biodegradation and not suitable for use in bioresistant MWFs. He says, “Sulfurized esters are highly biodegradable and should be used at a minimum in water-based bioresistant fluids. One other concern is that the extremely hazardous byproduct, hydrogen sulfide, will form during biodegradation of sulfurized additives. Phosphorus-based additives that are used as fertilizers also should be avoided. Some boron-based additives can contribute to boundary lubricity and greatly improve bioresistance.”
Butler suggests that some form of Four-Ball EP, Falex Pin and Vee Block, tapping torque or twist compression data, showing the potential performance of a specific boundary lubricity additive, will be useful to formulators. Andrews contends that the microtap test is the best option to correlate well with metal-removal applications such as drilling and tapping. But he believes that reliable lab tests do not exist for many applications, making it important for an additive supplier to provide actual machine data.
One of the challenges faced in evaluating boundary lubricity additives is to determine how any of the available lab tests correlate with field results. Andrews comments, “Certain lab tests do a decent job in giving a formulator a general idea if a particular boundary lubricity additive will perform well in a certain application. However, more often than not, something unexpected will arise during field trials that was not anticipated when lab test data was generated. A good rule to follow is to use lab data as a guide but rely more heavily on data produced during field trials.”
Butler recognizes that bench tests utilizing multiple alloys as test specimens correlate fairly well with real-world applications. He says, “Tests limited to specific ferrous test specimens are really only useful for predicting field results on similar ferrous alloys. A greater similarity between the lab test specimen and the field alloy will lead to better correlation in the test results. Alloy correlation trumps test contact geometry such as line, point or planar contact in predicting whether success in lab testing will translate into success in field trials.”
Future challenges
Lemke believes that the movement to light-weighting will be a prime motivator in the development of new boundary lubricity additives. He says, “Within the automotive and aerospace market segments, lightweight engineering is pushing all development, from new alloys to new materials/composites, new manufacturing processes and new technologies. MWFs have to keep up with the changing technologies using new additives in general and boundary lubricity additives in particular.”
Cheng sees that EH&S will be a pivotal factor in prompting formulators to move away from traditional boundary lubricity additives to new ones. He says, “Increasing sustainability requirements, wastewater treatment, biobased and higher efficiency will drive the MWF market to use more specialized additives that operate at low treat rates. Other factors that need to be considered include global registrations and the greater use of aluminum alloys and high strength steels in growing technologies such as electric vehicles (EVs).”
STLE member Marc Chan, marketing manager for Clariant, discusses the trends that will impact the evolution of MWFs into the future. He says, “New markets are rising such as the light metal parts in EV motors/drivers, and the MWF formulation will need to adapt to those changes. The evolution of minimum quantity lubrication means that boundary lubrication and film formation might be critical to the success of MWFs.”
Chan also stresses that sustainability will be a driver for formulators in the selection of additives in the future. He adds, “Now, more than ever, utilizing and identifying sustainable resources is a part of our everyday lives. MWFs are no exception as formulators will be looking for additives that are sustainable and renewable. Biostability, foam, oxidation and formulation stability are just a few of the properties that will need to be addressed when adjusting to the growing trend of sustainability.”
Lai believes that boundary lubricity additive selection in the future will be influenced by regulations taken to limit the use of traditional additives such as chlorinated paraffins. He says, “One of the most controversial boundary lubricity additives used in MWFs is chlorinated paraffins.
With all of the challenges faced in working with this EP agent, additive suppliers not only need to focus on developing boundary lubricity additives that can achieve the technical performance criteria required in metalworking but also to think one step ahead on any possible issue related to future regulations that might compromise the usage of specific additives such as chlorinated paraffins.”
Faber says, “Boundary lubricity additives will need to keep pace with advances in alloys and materials used in tomorrow’s manufacturing. Traditional EP additives are less effective on many of these materials, and formulators will be relying on boundary lubricity additives. Exciting advancements have been made in the development of water-based boundary lubricity additives that can be used in synthetic and semisynthetic MWFs, and more work needs to be done to explore their use in cutting and forming newer materials. Additive companies and finished fluid companies need to expand their use of new materials in their testing protocols, for example, including the use of titanium and compacted graphite iron in their tapping torque test regimen, and expanding the use of advanced high strength steel on metalforming tests such as cup draw tests. The knowledge gained from this expanded testing will accelerate advances in additive development and formulation design.”
As the MWF industry moves away from traditional boundary lubricity additives such as chlorinated paraffins, newer technologies are under development that combine the benefits of ester-based components in adsorbing to metal surfaces with the EP benefits found from using sulfur- and phosphorus-based derivatives. These technologies also are taken into consideration the growing move toward sustainability, with the knowledge that the MWF industry will be requiring additives that can perform at high levels but also meet more rigorous regulatory requirements.
Further information on boundary lubricity additives can be found in the following reference.
1
1.
Canter, N. (2017), “The chemistry of metalworking fluids,” in Byers, J., editor,
CRC Press, Boca Raton, Fla., pp. 150-153.