In contrast to table salt, which has an ordered arrangement of sodium and chlorine atoms, disordered rock salt oxides do not have a nice arrangement.
A disordered lithium vanadium oxide has been synthesized that exhibits potential to be as an alternative anode to graphite in lithium-ion batteries.
The performance of this disordered rock salt has been attributed to the lithium insertion mechanism.
Significant progress has been made in the development of lithium-ion batteries for use in applications such as electric vehicles. But challenges remain in developing a battery that exhibits excellent performance over a long period of time without the safety issues currently encountered.
One approach for identifying better options to use in lithium-ion batteries comes from the use of artificial intelligence (AI). In a previous TLT article,
1 researchers used this technique to identify potential substances as battery electrolyte candidates. Electrolytes have been found to be a source of instability because they can facilitate the formation of dendrites, leading to battery short circuiting. Alternative electrolyte candidates were examined by evaluating the enthalpy of formation of 133,000 organic molecules selected from a database. Enthalpy of formation was chosen by the researchers because it is a measure of molecular stability.
Another source of the instability in the lithium-ion battery is graphite, which is the most commonly used anode. Ping Liu, professor at the Center for Memory and Recording Research at the University of California (UC) San Diego in La Jolla, Calif., says, “The problem with graphite is the voltage potential for this material is less than the voltage potential for lithium. When a battery is undergoing charging, this issue will lead to the plating of lithium metal on the graphite anode causing loss of battery cycle life and potentially leading to instability.”
In an effort to evaluate other electrode options, Liu and his colleague, Shyue Ping Ong, associate professor of nanoengineering at UC San Diego, turned to studying disordered rock salt oxides. Ong says, “Ordinary table salt has an ordered arrangement of sodium and chlorine atoms in a specific geometry. In contrast, disordered rock salt oxides do not have such a nice arrangement. By substituting oxygen for chlorine atoms and lithium and vanadium atoms in a ratio of 60:40 for sodium atoms, an interesting disordered rock salt oxide with the structure Li
3V
2O
5 is formed.”
Disordered rock salts have been known to researchers developing lithium-ion batteries because they have large capacities for storing lithium. Liu says, “There continues to be a push for ultrafast charging batteries. Users stress that batteries are not charging fast enough for applications such as cell phones. In the past, disordered rock salts have been examined for use as the cathode in lithium-ion batteries.”
Liu and Ong decided to evaluate the potential for using this disordered lithium vanadium oxide as an anode material because they felt the crystal structure will be able to incorporate more lithium ions, which will enhance the operational capacity of the battery.
 Ordinary table salt (shown above) has an ordered arrangement of sodium and chlorine atoms in a specific geometry. In contrast, disordered rock salt oxides do not have such a nice arrangement. By substituting oxygen for chlorine atoms and lithium and vanadium atoms in a ratio of 60:40 for sodium atoms, an interesting disordered rock salt oxide is formed.
Lithium insertion/ redistribution mechanism
Ordinary table salt (shown above) has an ordered arrangement of sodium and chlorine atoms in a specific geometry. In contrast, disordered rock salt oxides do not have such a nice arrangement. By substituting oxygen for chlorine atoms and lithium and vanadium atoms in a ratio of 60:40 for sodium atoms, an interesting disordered rock salt oxide is formed.
Lithium insertion/ redistribution mechanism
Liu, Ong and their colleagues synthesized the disordered lithium vanadium oxide, evaluated its crystal structure and determined through experimentation that it has potential for use as an anode in lithium-ion batteries. The disordered rock salt was prepared through lithiation of vanadium oxide using butyl lithium under an argon atmosphere for 60 minutes at ambient temperatures.
Initial attempts to use X-ray diffraction to categorize the disordered rock salt proved to be difficult. Liu says, “The disordered rock salt exhibited a highly symmetric crystal structure, which means that very few peaks are detected. Lithium atoms also cannot be imaged by X-rays.”
The researchers decided to use neutron diffraction in combination with X-ray diffraction to provide some structural information. Further information was obtained through the use of high-resolution scanning transmission electron microscopy. The structure of the disordered lithium vanadium oxide is shown in Figure 2. A key structural feature for the disordered rock salt is the presence of lithium atoms by themselves in tetrahedral sites and in combination with vanadium atoms in octahedral sites.
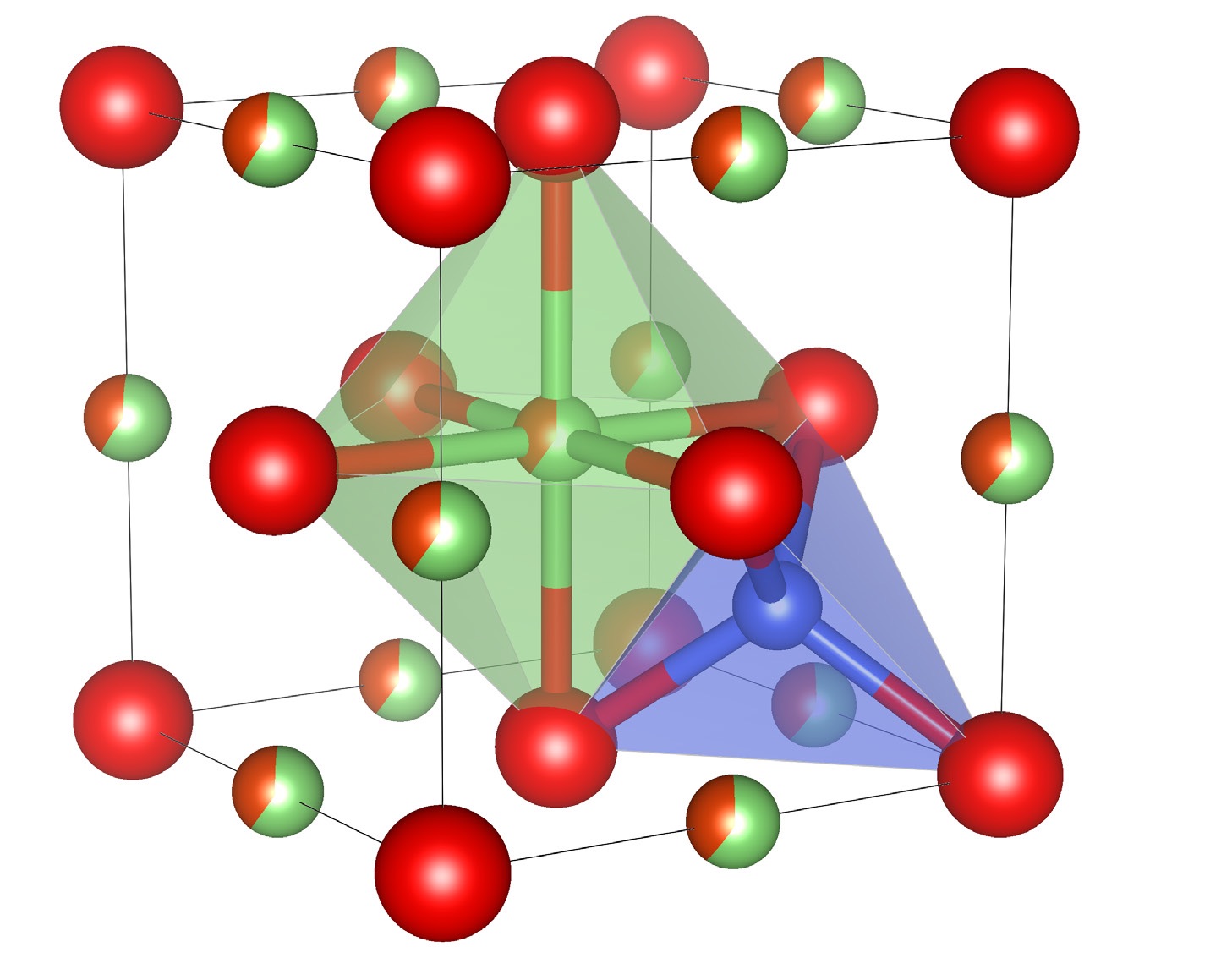 Figure 2. A schematic of the crystal structure of the disordered lithium vanadium oxide is shown. The red balls represent oxygen atoms, the blue tetrahedron represents lithium in tetrahedral sites, and the green octahedron represents the lithium/vanadium shared octahedral sites. Figure courtesy of UC San Diego.
Figure 2. A schematic of the crystal structure of the disordered lithium vanadium oxide is shown. The red balls represent oxygen atoms, the blue tetrahedron represents lithium in tetrahedral sites, and the green octahedron represents the lithium/vanadium shared octahedral sites. Figure courtesy of UC San Diego.
The performance of the disordered rock salt was determined by testing with a lithium-metal counter electrode and then in a full cell using a conventional cathode based on a lithium, nickel, manganese, cobalt oxide. Two benefits that stand out from the testing is good durability through 1,000 charge-discharge cycles and exceptional rate capacity over a short period of time. The disordered rock salt can utilize 40% of its capacity within 20 seconds.
Liu says,” The performance of the disordered lithium vanadium oxide is superior to lithium titanate, an alternative anode to graphite that supports fast charging but loses too much energy density. This disordered rock salt also is safer to use than graphite, reducing the possibility of battery failure.”
Liu indicates that the difference in the voltage capacity between the disordered lithium vanadium oxide and lithium adds a safety factor minimizing the possibility of the metal plating on the anode. He says, “Another benefit is the disordered rock salt exhibits a very small volume charge (5%-6%) during charge/discharging cycles in comparison to graphite. This small change in volume leads to the formation of a very stable solid electrolyte interphase layer. The result is a very stable anode that will lead to an added safety margin for the battery.”
Ong indicated that the reason for the benefits of the disordered rock salt is due to the lithium insertion mechanism. He says, “In the disordered rock salt anode, all of the octahedral sites are occupied. When lithium atoms enter the anode, lithium is initially placed in tetrahedral sites. But as the tetrahedral sites fill up, electrostatic repulsion increases causing other lithium atoms already present in octahedral sites to be displaced into tetrahedral sites. The energy barrier for such displacements is low and results in high rate performance.”
The researchers hope to improve the performance of the disordered rock salt in the future. Liu says, “To utilize the disordered lithium vanadium oxide in ultrafast charging applications such as power tools, we are looking to improve its synthesis and increase its electrical conductivity. Ong adds, “We will be trying to gain a better understanding of this new insertion mechanism. Among the questions to be answered is, why is this material so stable, and what is the role of disorder?”
Additional information can be found in a recent article
2 or by contacting Liu at
piliu@eng.ucsd.edu or Ong at
ongsp@eng.ucsd.edu.
REFERENCES
1.
Canter, N. (2020), “Using AI to identify battery electrodes,” TLT,
76 (3), pp. 14-15.
2.
Liu, H., Zhu, Z., Yan, Q., Yu, S., He, X., Chen, Y., Zhang, R., Ma, L., Liu, T., Li, M., Lin, R., Chen, Y., Li, Y., Xing, X., Choi, Y., Gao, L., Cho, H., An, K., Feng, J., Kostecki, R., Amine, K., Wu, T., Lu, J., Xin, H., Ong, S. and Liu, P. (2020), “A disordered rock salt anode for fast-charging lithium-ion batteries,”
Nature, 585 (7823), pp. 63-67.