Clathrates: New type of superdiamond
Dr. Neil Canter, Contributing Editor | TLT Tech Beat April 2020
The addition of boron stabilized the clathrate and made it easier to accommodate guest atoms.
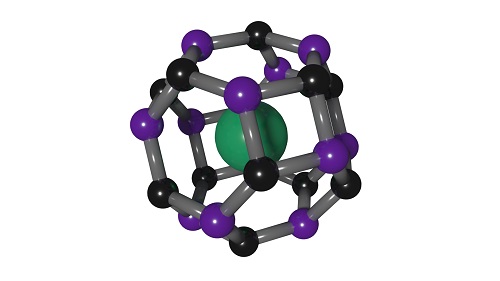 Figure 3. The unit cell of a new clathrate with properties similar to diamond has the strontium atom in green, boron atoms in black and carbon atoms in purple. Figure courtesy of the Carnegie Institution for Science.
Figure 3. The unit cell of a new clathrate with properties similar to diamond has the strontium atom in green, boron atoms in black and carbon atoms in purple. Figure courtesy of the Carnegie Institution for Science.
KEY CONCEPTS
- A clathrate based on strontium, boron and carbon displays similar mechanical properties to diamond.
- Boron was introduced because its electronic structure improves the stability of the carbon-based clathrate.
- Varying the guest atom in the clathrate cage may enable this new class of compounds to have tunable electronic structures.
Diamond is a material known for high hardness, good thermal conductivity and high electron mobility. In fact, diamond has a thermal conductivity that is five times better than copper.
Diamond is also a material that is representative of a class of three-dimensional sp3 carbon-based structures. The challenge for researchers is that many comparable compounds have been predicted theoretically but few have been actually synthesized.
Tim Strobel, staff scientist at the Carnegie Institution for Science in Washington, D.C., says, “There are many examples of sp3-based carbon compounds including a plethora of organic compounds. Besides diamond, only a limited number of three-dimensional sp3 carbon-based structures have been produced including lonsdaleite (a hexagonal diamond allotrope), boron-doped diamond and boron, nitrogen substituted diamond-like structures such as BC2N.”
One of the reasons that three-dimensional sp3 carbon-based structures are difficult to produce is that high temperature and pressure conditions are required. One technique is to place the reactants in a diamond anvil cell under gigapascal pressures and to use a laser heat technique to reach temperatures approaching 2500 K.
In a previous TLT article (1), similar conditions were used to produce sodium polyhydrides, which are compounds that contain multiple hydrogen atoms. The compound produced contained hydrogen molecules within the polyhydride structure. Researchers believe that polyhydrides are precursors to hydrogen-based superconductors.
An interesting three-dimensional sp3 carbon-based structure that has not been previously synthesized is a carbon-based clathrate. Strobel says, “Clathrates are extended open-frame structures that contain polyhedral cages. Theoretical studies suggest that carbon clathrates may demonstrate hardness comparable to diamond but exhibit superior mechanical properties. An interesting characteristic of clathrates is their ability to trap guest atoms in the open framework structure. This leads to the potential to develop many different clathrate derivatives with tunable properties.”
The problem in attempting to synthesize carbon clathrates is their thermodynamic instability. A new approach has now been taken to synthesize three-dimensional sp3-based carbon clathrates by substituting some of the carbon atoms with boron.
SrB3C3
Strobel and his colleagues synthesized a clathrate for the first time that is based on a carbon-boron cubic bipartite sodalite structure that contains sp3-bonded truncated octahedral host cages. The empirical composition of the host cage is B3C3 with a Sr2+ guest cation trapped as a guest atom.
Strobel says, “Pure clathrates contain cages that need some type of guest atom to be placed inside for synthesis. Traditionally, this atom has been from the alkali, alkali earth or halogen series classes. Filled clathrates based on carbon should contain extra electrons within unoccupied antibonding states on the framework. For this reason, filled clathrates based on pure carbon have not been thermodynamically stabilized and, in all probability, are extremely difficult to synthesize.”
The researchers, recognizing this challenge, decided to evaluate the substitution of some of the carbon atoms with boron. Strobel says, “Our approach was to evaluate the substitution of boron for carbon atoms because boron is one electron deficient, and able to interact with extra electrons donated from the guest atoms, improving the stability of the clathrate. Another advantage is that the boron-carbon bond is longer than the carbon-carbon bond. This means a larger cage with boron atoms will form, making it easier to accommodate guest atoms.”
After the researchers conducted a theoretical study evaluating the best stoichiometry for a carbon-boron clathrate using an Advanced Structure Algorithm (that employs artificial intelligence), the most thermodynamically stable structure identified is B3C3.
The next step for the researchers was to identify a suitable guest atom. Strobel says, “Of the elements evaluated in the first and second row of the periodic table, we found that strontium enabled us to produce the most stable clathrate at the lowest pressure.”
The clathrate based on strontium, boron and carbon was synthesized from SrB6, SrC2 and carbon. Powders of these three raw materials were added to a diamond anvil cell and compressed in neon or aluminum oxide media to a pressure over 50 gigapascals. An infrared laser was used to heat the mixture to 2500 K and the reaction was followed by x-ray diffraction.
The x-ray diffraction pattern obtained matched a calculated pattern for SrB3C3. Further annealing under high-pressure and high-temperature conditions produced crystalline grains. Single-crystal diffraction confirmed the finding and allowed for a more thorough characterization of the unit cell for SrB3C3.
Figure 3 shows a unit cell where the strontium atom is in green, boron atoms are in black, and carbon atoms are in purple. The clathrate is only thermodynamically stable at high pressure though the researchers report that it can be recovered at atmospheric pressure if kept under an inert atmosphere. But degradation takes place over a few hours upon exposure to moisture in the air.
The researchers show that this new material exhibits a high bulk modulus (incompressibility similar to B4C), and an estimated Vickers hardness comparable to tungsten carbide.
Strobel says, “SrB3C3 displays the properties of a metal and samples isolated have a metallic luster. We anticipate that this material may also exhibit superconductivity at notably high temperatures.”
The researchers recognize that this new clathrate that has mechanical properties similar to diamond with the advantage of a tunable electronic structure. Strobel says, “We anticipate incorporating other guest atoms such as lanthanide in the cage in the future. Other elements such as nitrogen may also be substituted for boron in the cage in an effort to find a clathrate that is stable under ambient conditions.
Further information can be found in a recent article (2) or by contacting Strobel at tstrobel@carnegiescience.edu.
REFERENCES
1. Canter, N. (2016), “Sodium polyhydrides: Potential superconductors,” TLT, 72 (11), pp. 10-11.
2. Zhu, L., Borstad, G., Liu, H., Gunka, P., Guerette, M., Dolyniuk, J., Meng, Y., Greenberg, E., Prakapenka, V., Chaloux, B., Epshteyn, A., Cohen, R. and Strobel, T. (2020), “Carbon-boron clathrates as a new class of sp3-bonded framework materials,” Science Advances, 6 (2), eaay8361.
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat can be submitted to him at neilcanter@comcast.net.