Aqueous lithium-ion battery
Dr. Neil Canter, Contributing Editor | TLT Tech Beat March 2020
RPI researches potential cathode replacement materials.
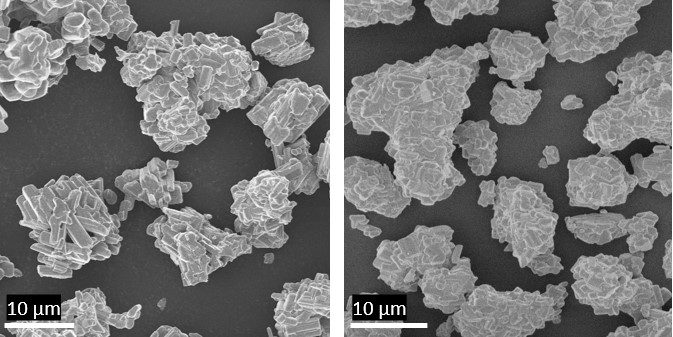 Figure 1. Scanning electron microscopy images of the two niobium tungsten oxides evaluated for use as anodes in an aqueous lithium-ion battery. (Figure courtesy of Rensselaer Polytechnic Institute.)
Figure 1. Scanning electron microscopy images of the two niobium tungsten oxides evaluated for use as anodes in an aqueous lithium-ion battery. (Figure courtesy of Rensselaer Polytechnic Institute.)
Figure Courtesy of Rensselaer Polytechnic Institute
KEY CONCEPTS
•
An aqueous lithium-ion battery was developed using niobium tungsten oxide as the anode and a water-in-salt electrolyte.
•
Niobium tungsten oxide was chosen as the anode because it has the potential to generate high volumetric energy densities and has well-defined channels to facilitate diffusion of lithium ions.
•
The volumetric performance observed was the best seen to date for an aqueous lithium-ion battery.
Ongoing development of lithium-ion batteries is enabling researchers to steadily improve their performance and durability. A focus point has been to find approaches for improving the graphite anode. But work has also been underway to find alternative materials for the cathode.
In a previous TLT article
1, researchers identified vanadium disulfide as a potential cathodic material. This material was chosen because it is electrically conductive and has two important benefits (lighter in weight and environmentally safe) as compared to the incumbent cathode, lithium cobalt oxide. Inherent instability in the vanadium disulfide cathode was overcome through application of a thin layer of titanium disulfide.
Safety issues continue to be a significant hurdle for lithium-ion batteries. Nikhil Koratkar, professor of mechanical, aerospace and nuclear engineering at Rensselaer Polytechnic Institute in Troy, N.Y., says, “The existing lithium-ion battery requires the use of a non-aqueous, organic solvent-based electrolyte. This material is the source for concerns about flammability, moisture sensitivity and toxicity. Fabrication of the battery is more expensive because safeguards must be put in place when using a solvent based electrolyte.”
An option to examine is replacement with an alternative that is compatible with water. Koratkar says, “The problem with using an aqueous electrolyte is that the theoretical applied voltage is limited to less than 1.23 volts. Above that limit, water will electrolyze to form hydrogen and oxygen. In actuality, voltages between 1.6 and 1.8 Volts can be achieved without water decomposition.”
Recent work by another research group has provided an aqueous electrolyte composition that is resistant to electrolysis and enables the resulting battery to exhibit a high energy density. Koratkar says, “This composition, known as a water-in-salt electrolyte, is readily water soluble and forms a gel that contains a high concentration of salt with a limited amount of water.”
Among lithium-containing salts, only lithium bis (trifluoromethane sulfonyl) imide has a high enough solubility in water to be used for the water-in-salt electrolyte.
In theory, an aqueous lithium-ion battery should exhibit superior rate performance because aqueous electrolytes have higher ionic conductivity than organic solvents. But in actuality this has not been the case.
Koratkar believes that one of the challenges is to optimize the anode and the cathode used with the aqueous electrolyte. He says, “A cathode based on lithium manganese oxide is effective, but finding a suitable anode has been challenging. Graphite is not suitable for use as an anode because this material is hydrophobic and not compatible with aqueous systems.”
A new anode has been identified and evaluated in an aqueous lithium-ion battery.
Niobium tungsten oxide
Koratkar says he and his colleagues evaluated niobium tungsten oxides as a potential anode candidate for two reasons: “Being oxides, these materials hydrogen bond with water and hence exhibit good hydrophilicity. They also exhibit well-defined channels which facilitate the diffusion of lithium ions leading to fast charging.”
The niobium tungsten oxides do contain two heavy elements (niobium and tungsten), but these materials are very densely packed, leading to the ability of the researchers to produce a very dense anode electrode. This means that niobium tungsten oxides have the potential for generating high volumetric energy densities.
Particles between 20 and 30 microns in size were used by the researchers and are shown in Figure 1.
The researchers evaluated two niobium tungsten oxides that have the following structures: Nb
18W
16O
93 and Nb
16W
5O
55. Both species were evaluated in a full-cell aqueous lithium-ion battery by cyclic voltammetry using the lithium-based water-in-salt electrolyte and a lithium manganese oxide-based cathode.
Koratkar says, “We focused on evaluating the volumetric energy density as opposed to the gravimetric energy density which has been more widely used in academia. But for consumer electronics, electric vehicles and grid storage industries, volumetric energy density is more relevant. The high density of niobium tungsten oxides means that the anode stores a large amount of energy in a small volume.”
In evaluating the two niobium tungsten oxides, the researchers determined that the species with eighteen niobium and sixteen tungsten atoms exhibited better performance in the aqueous electrolyte. Koratkar says, “Nb
18W
16O
93 is more effective than Nb
16W
5O
55 because it contains a greater number of tungsten ions per formula unit. Under the restrictive voltage window for the aqueous electrolyte, tungsten is more dominant in the reduction reaction than niobium.”
For a traditional non-aqueous electrolyte containing battery, the opposite takes place. Koratkar says, “The oxide with a higher percentage of niobium performs better because this atom can more effectively undergo reduction at the higher voltage possible in the non-aqueous battery.”
The researchers found that the more effective niobium tungsten oxide exhibited a volumetric rate of approximately 200 ampere hours/ liter at a 1C rate which is higher than graphite. Battery cycling could also be conducted at a higher rate with only a 25% reduction in capacity detected.
The volumetric performance observed by the researchers was the best seen to date for an aqueous lithium-ion battery. For the future, the researchers will be trying to extend the electrochemical stability voltage window so the aqueous lithium-ion battery can compete more effectively with non-aqueous batteries.
Koratkar says, “We will be trying to optimize the electrolyte which is currently being used at a concentration of 21M. Moving to a higher concentration may reduce the amount of water in the electrolyte increasing the voltage window but also increasing cost. Evaluating lower electrolyte concentrations will also be tried to see how the voltage window is affected with a higher concentration of water.”
Koratkar also indicates that other electrolytes will be evaluated. He adds, “The anode will be optimized by replacing niobium and tungsten with lighter weight elements in the form of other oxides or through doping.”
Additional information on this research can be found in a recent article
2 or by contacting Koratkar at
koratn@rpi.edu.
REFERENCES
1.
Canter, N. (2019), “Potential new cathode material for a lithium-ion battery,” Tribology & Lubrication Technology,
75 (7), pp 16- 17
2.
Lakhnot, A., Gupta, T., Singh, Y., Hundekar, P., Jain, R., Han, F. and Koratkar, N. (2019). “Aqueous lithium-ion batteries with niobium tungsten oxide anodes for superior volumetric and rate capability,”
Energy Storage Materials, click
here.
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat can be submitted to him at neilcanter@comcast.net.