KEY CONCEPTS
•
Aluminum staining is caused by a number of factors including high pH that leads to the dissolution of the protective oxide layer coating the metal.
• Three different mechanisms that aluminum stain inhibitors utilize to protect the metal are chemisorption, formation of complexes with corrosive agents and a protective film acting as a physical barrier
• The recommended test method is to immerse aluminum coupons into fully formulated metalworking fluids at alkaline pH values (at a minimum of 9.2).
• Future demand for more effective corrosion/stain inhibitors will increase due to the growing use of aluminum in combination with other materials.
The ongoing drive to improve efficiency has led end-users to produce machinery that is lighter in weight. A case in point is the automotive industry where original equipment manufacturers (OEMs) are facing the challenge of complying with more challenging fuel economy and emissions regulations.
This trend has led OEMs to incorporate lighter weight alloys into their vehicles. A key example is aluminum. In 2015 the Ford Motor Co. decided to transform its best-selling F-150 pickup truck from a steel to an aluminum alloy body (
1). This process reduced the weight of the newly designed truck by more than 300 kilograms. The alloy used by Ford is a 6000 series wrought aluminum.
The result of the move by Ford and other end-users is an increase in the need for metalworking fluids (MWFs) that can be used to form and machine aluminum alloys and also have the versatility to form and machine other alloys such as steel and copper. But there are challenges faced by MWF in working with aluminum. One of those challenges is understanding and minimizing the degree of staining that can be seen on fabricated aluminum parts.
The purpose of this article is to discuss the origin of aluminum staining and to discuss what approaches can be taken to minimize it. Input on the issue was obtained from industry experts who have perspectives from the additive and formulator standpoints. The following individuals were contacted: Harish Potnis, ANGUS Chemical Co., Stephanie Cole, Clariant, Dr. Yu-Sen Chen, Dober Chemical, William Harwood, Italmatch Chemicals Group, Jennifer Lunn, JTM Products Inc., Dr. Britt Minch, The Lubrizol Corp., Kevin Saunderson, New Age Chemical.
Cause of aluminum staining
STLE-member William Harwood, global product manager, Water Based MWF for Italmatch SC, LLC in Cleveland, identifies three causes of aluminum staining. He says, “Aluminum is naturally protected from corrosion since it reacts with oxygen forming a stable oxide layer. Under alkaline conditions present in water-based metalworking fluids, the oxide layer dissolves, and aluminum can show signs of corrosion/staining which range from light yellow to very dark gray. A second cause is the occurrence of galvanic corrosion when two dissimilar metals are in contact in an aqueous environment. For example, while machining aluminum that is in contact with the cast iron/steel bed of the machine tool, aluminum will tend to corrode in preference to the steel alloy. A third cause is high chloride levels (that may be present in water used in machining operations) increasing the chances for aluminum staining.”
STLE-member Stephanie Cole, formulation chemist with Clariant in Mt. Holly, N.C., also lists three causes for aluminum staining. She says, “Aluminum staining can be caused by filiform corrosion, galvanic corrosion and poultice corrosion. Filiform corrosion is caused by the imperfections of other metals used in various aluminum alloys. Galvanic corrosion happened upon exposure of aluminum to other metals in the presence of electrolytes such as salt water. Poultice corrosion is the process that occurs when the natural aluminum oxide layer degrades.”
STLE-member Dr. Britt Minch, research manager, Metalworking, for The Lubrizol Corp. in Wickliffe, Ohio, says, “Aluminum and its alloys are usually not the first metallurgy that comes to mind when discussing corrosion. Protective oxides cover aluminum surfaces and protects the metal surface from the environment. Corrosive ions such as chlorides and sulfates can penetrate the protective layer leading to pitting. Metalworking fluids are generally formulated at a pH greater than 9 to minimize both ferrous corrosion and biological activity. As a consequence of the high pH, the protective oxide layer is etched away by the metalworking fluid. One of the sensitivities of aluminum machining is its tendency to stain at high pH.”
STLE-member Dr. Yu-Sen Chen, R&D director for Dober Chemical in Woodbridge, Ill., says, “The destruction and/or dissolution, of aluminum’s protective oxide film and or/other protective film/layer on the aluminum surface causes staining.”
STLE-member Harish Potnis, global technical manager, Metalworking Fluids, for ANGUS Chemical Co. in Buffalo Grove, Ill., indicates that aluminum corrosion/staining is the result of metal corrosion during the metalworking fluid process. He says, “Aluminum staining can be influenced by several common negative factors that include high pH, alkalinity, specific acids and water quality.”
Aluminum stain inhibitors
Minch states that there are a variety of chemistries available on the market today that can be used to protect aluminum alloys. He says, “The chemistries differ greatly in what they can offer in terms of performance across a range of fluid types, water hardness levels (for water-based fluids), pH ranges and even metallurgies. Phosphorus-based corrosion inhibitors are among the most effective inhibitors because they have broad applicability across all fluid types and pH ranges. The phosphorus head group has an extremely strong affinity for the aluminum surface. Many different types of phosphorus-based inhibitors are available, some of which are optimized to provide surfactancy, improve metalworking fluid hard water compatibility and/or improve fluid longevity.”
Minch continues by discussing other inhibitors based on sulfonates, carboxylic acid/amine salts, polymers and silicates. He says, “Certain amino sulfonates have been used as corrosion inhibitors for aluminum alloys, but they tend to be more limited in terms of effective pH range. The sulfonate-based inhibitors tend to be limited to fluids with significant concentrations of oil to allow for their solubilization. Carboxylic acid/amine salts can be useful in certain conditions but tend to be most limited in effective pH range and are only useful in lower water hardness levels. A few polymeric inhibitors that are available in the market can be quite effective in emulsifiable oils or high-oil semi-synthetic metalworking fluids, however, they tend not to be compatible in synthetic fluids. Finally, silicates as sodium or potassium salts are commonly used inorganic corrosion inhibitors for aluminum alloys. Their main drawbacks are they tend to be used at high pH values (>9) and may leave an undesirable, tacky residue on the workpiece or the machine tool.”
Potnis feels that while several aluminum corrosion inhibitors are available, certain formulary and regulatory requirements often can impact their selection and use. He says, “Some of the most common chemistries include sodium silicates, phosphate esters, amine salts and amine carboxylates. Silicates, while readily available, can be difficult to incorporate into a formulation and can eventually precipitate over time leading to filter blockage. Phosphate esters are widely used as extreme pressure (EP) additives, but not all types are good stain inhibitors, and they are known to encourage microbial growth. Amine salts and amine carboxylates can be effective, but water hardness and chloride variation can be problematic.”
Harwood lists three types of aluminum stain inhibitors: phosphates, silicates and triazoles/thiadiazoles. He says, “Phosphates in the form of phosphate esters are effective but can be prone to fungal attack and restricted upon disposal of the depleted water-based metalworking fluids. Those phosphate esters containing higher ethylene oxide levels may also generate high levels of foam which is not desirable. Silicates can also inhibit aluminum staining but may not be compatible with the metalworking fluid formulation. All of these inhibitors are typically used at low treat rates (about 0.5%) in the metalworking fluid concentrate.”
Cole divides aluminum stain inhibitors into two categories: inorganic and organic based. She says, “Inorganic stain inhibitors are available but require additional processing and a change of surface profiling. In contrast, organic types can be applied simply but are temporary at best.”
How inhibitors function
Aluminum stain inhibitors function via three different mechanisms (chemisorption, complex and protective film) according to Potnis. He says, “The chemisorption principle involves the inhibitor forming a protective layer via a chemical reaction/bond on the metallic surface. In solution, an aluminum stain inhibitor can form a complex with corrosive agents preventing them from staining the metal surface. A physical barrier can form a protective film to protect the base metal from staining.”
Cole differentiates the approaches used by inorganic and organic aluminum stain inhibitors. She says, “Inorganic corrosion inhibitors create a chemically bonded barrier, while organic inhibitors produce a hydrophobic film that promotes water repellency.”
Harwood provides insight on how inhibitors use an adsorption process. He says, “Most inhibitors that use a surface adsorption mechanism where they are capable of donating a pair of electrons through the presence of oxygen, nitrogen, phosphorus and sulfur atoms in these molecules. Silicates do not operate using this mechanism but rather provide stain inhibition through a chemisorption process.”
Chen says, “Aluminum stain inhibitors either form a strong and adherent oxide film, form a protective layer or chemically adsorb to the metal surface.”
Minch feels that the mechanism of aluminum protection will vary depending on the chemistry used as the inhibitor. He says, “For example, phosphorus-based corrosion inhibitors chemically adsorb to the metal surface leaving a thin barrier layer to protect the underlying metal surface. Regardless of the inhibitor type, only a few layers of the inhibitor will attach to the surface and organize to form a protective layer, thus the transient nature of these types of corrosion inhibitors.”
Performance requirements
Cole believes an aluminum stain inhibitor must be effective in a variety of formulations and yet not interfere with other additives required in complex MWF formulations. She says, “An aluminum stain inhibitor must work in a wider range of metalworking fluid types but not disrupt the formulation stability, not disrupt ferrous metal protection and not reduce reserve alkalinity.”
Minch says, “When choosing an aluminum stain inhibitor, there are multiple performance parameters that need to be taken into consideration. First and foremost, the main decision point for a stain inhibitor is whether it will provide the desired level of corrosion protection for all metal alloys that are expected to encounter the fluid. The pH of the metalworking fluid many times will limit the choices available to the formulator. Long-term fluid stability may also be a driver in the choice of inhibitor. For example, silicates may precipitate out of solution or even plate out on the workpiece, lowering the effectiveness of the inhibitor over time. The formulator would also be wise to look at hard water stability of the fluids to ensure that the stain inhibitor does not have any negative impact.”
Chen focuses requirements for an aluminum stain inhibitor on effectiveness and performance. He says, “Dosage, performance under different corrosive environments and effective pH range are some of the criteria that need to be examined in selecting an aluminum stain inhibitor. Performance of the inhibitor should be evaluated in a complete formulation in the presence of other inhibitors and components. Other factors to consider are ease and stability in formulating, meeting environmental requirements and availability.”
Potnis indicates there are two important considerations to ensuring performance across all applications: compatibility with other formulator components and multi-metal compatibility. He says, “Additional performance considerations include but are not limited to, functionality in elevated pH environments, vapor phase corrosion performance and impact on microbial growth.”
Harwood states that a formulator making a selection must keep in mind formulation compatibility and inhibitor stability. He says, “The inhibitor should be compatible with the formulation that is being prepared. Ensure that the formulation is inherently low staining/low pH first, then add the inhibitor. Some inhibitors are susceptible to hard water (calcium and magnesium water salts) and can precipitate out of solution. Some inhibitors are not stable in concentrates which have a water content above 5%.”
Amines
Amines fulfill a number of important functions in a MWF, but aluminum stain inhibition is not one of them. Minch discusses why formulators need to consider what amines to work with in minimizing staining. He says, “Alkanolamines are commonly used in metalworking fluids to raise the pH of the fluid and to salt acidic components. These alkanolamines are a critical tool in the formulator’s toolbox, but they come at a price. While these stronger amines are often needed to help solubilize other components in the formulation and adjust the pH to the desired range, their pKa value’s boost the pH of the metalworking fluids, resulting in more aluminum staining and residues.”
Potnis discusses specific amines that have been found to minimize aluminum staining. He says, “3-Amino-4-octanol (3A40) and 2-amino-1-butanol/2-amino- 2-ethylpropanediol (AB/AEPD) can be beneficial. These chemistries are effective at high pH values with (AB/AEPD promoting better neutralization efficiency. The performance of these alkanolamines alone, as well as in combination with other amines, has been studied extensively and performance benefits have been seen on widely used aluminum alloys such as 356, 2024, 6061 and 7075.”
Screening tests
All respondents indicate that the best approach for evaluating staining is to immerse aluminum metal coupons in a specific fluid. Harwood says, “Typical tests are done on the diluted fluid (at working concentration, e.g. 5%) and a series of aluminum alloys are immersed in the fluids for a period of time. Once removed, the coupons are evaluated for change in appearance and also weight gain or loss compared to the test coupon. Sandwich tests are also conducted in some aerospace approvals. These procedures are more focused on galvanic corrosion.”
 Figure 1. Evaluation of four aluminum and one copper alloys in a 60% oil containing emulsifiable oil are shown. Results from testing with (bottom row) and without (top row) a phosphate ester show the importance of using this additive to minimize staining. (Figure courtesy of Italmatch SC, LLC.)
Figure 1. Evaluation of four aluminum and one copper alloys in a 60% oil containing emulsifiable oil are shown. Results from testing with (bottom row) and without (top row) a phosphate ester show the importance of using this additive to minimize staining. (Figure courtesy of Italmatch SC, LLC.)
 Figure 2. Staining/corrosion/pitting is observed from aluminum coupons soaked in metalworking fluid diluted to between 3% and 5% in 20 dh (approximately 350 ppm) hard water at 50 C for 18 hours. (Figure courtesy of Clariant.)
Figure 2. Staining/corrosion/pitting is observed from aluminum coupons soaked in metalworking fluid diluted to between 3% and 5% in 20 dh (approximately 350 ppm) hard water at 50 C for 18 hours. (Figure courtesy of Clariant.)
 Figure 3. Seven pairs of aluminum coupons were fully immersed and evaluated in a metalworking fluid at a pH of 9.2. The coupon on the left was immersed in a fluid containing a phosphorus-based corrosion inhibitor while the right coupon was immersed in the same fluid without the corrosion inhibitor. (Figure courtesy of The Lubrizol Corp.)
Figure 3. Seven pairs of aluminum coupons were fully immersed and evaluated in a metalworking fluid at a pH of 9.2. The coupon on the left was immersed in a fluid containing a phosphorus-based corrosion inhibitor while the right coupon was immersed in the same fluid without the corrosion inhibitor. (Figure courtesy of The Lubrizol Corp.)
Figure 1 shows an example of the type of testing Harwood believes needs to be done. A 60% oil containing emulsifiable oil is evaluated at a 5% concentration for three hours at 55 C with the four aluminum and one copper alloys listed. The emulsifiable oil is formulated with (pH 9.2) and without a phosphate ester (pH 9.3). Inclusion of the phosphate ester in the formulation led to the elimination of staining in all five alloys tested.
Cole’s approach is to soak aluminum panels in 20 dH (approximately 350 ppm) hard water in a MWF diluted to a concentration between 3% and 5%. She says, “Be sure the coupon is halfway submerged to evaluate the vapor phase of the aluminum coupon. Enclose the container and place it in a 50 C oven overnight (18 hours). In the morning, observe the staining/corrosion/pitting (
see Figure 2). Be sure to include two controls of 100% deionized water and 100% of the 350 ppm hard water without any metalworking fluid. Please keep in mind that this is a very harsh test.”
For evaluation of Filiform corrosion, Cole advises that standards can be obtained from ASTM D2803 (Standard Guide for Testing Filiform Corrosion Resistance of Organic Coatings on Metal) (
2).
Minch says, “The most common way to quickly screen the efficacy of a corrosion inhibitor is simply by fully or partially immersing a single metal coupon of the desired alloy in the test fluids and allowing it to sit at room temperature for 24 to 48 hours. The severity of the test can be increased by elevating the temperature to 40 C or 50 C. Upon removal from the fluid, the coupon can be visually inspected for staining. Both the immersed portion and the portion of the coupon above the fluid may be of interest (if only partially immersed). Ideally, the metal surface will be free of black or brown oxidation stains. Coupons are often monitored for weight loss or gain. Screening tests come in a variety of forms, but the best test is always the one that most closely emulates the conditions that the metal and fluid experience in the field.”
Figure 3 shows the results from an immersion test for seven pairs of aluminum coupons immersed in a MWF at a pH of 9.2. The difference in the composition of the metalworking fluid is those coupons immersed in a fluid containing a phosphorus-based corrosion inhibitor are on the left, while results from coupons immersed in the same fluid without the corrosion inhibitor are on the right.
Potnis feels that both liquid and vapor phase aluminum staining performance should be conducted at a minimum in varying degrees of water hardness/chloride levels. He says, “Ideally, the formulation also should be evaluated for microbial performance if this is a fluid that will be recirculated or has potential for microbial contamination. Of course, the emulsion must also be stable and other performance attributes such as cast-iron corrosion, must also meet necessary specifications.”
Phosphate free formulations containing a triazine biocide were evaluated at a pH of 10 in coupon testing with 6061 Aluminum. The evaluation was performed on metalworking fluids diluted to 5% in 200 ppm water hardness. The fluids were prepared with different amines (
see caption for Figure 4 for identification of the amines). Top and bottom results shown in Figure 4 differed due to the addition of 1% 3A40 for the bottom set of results.
Chen recommends two types of screening tests. He says, “An aluminum stain inhibitor should be evaluated for weight loss from a coupon test which is a chemical test. Electrochemical techniques such as polarization and electrochemical impedance spectroscopy also should be used.”
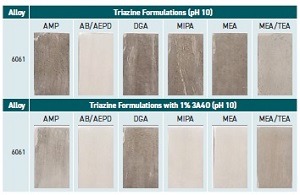 Figure 4. Phosphate free MWF formulations containing a triazine biocide were evaluated at a pH of 10 in coupon testing at a 5% dilution in 200 ppm water hardness. Fluids were tested in the following amines: AMP (2-amino-2-methyl-1-propanol), AB/AEPD (2-amino-1-butanol/2-amino-2-ethylpropanediol), DGA (diglycolamine), MIPA (monoisopropanolamine), MEA (monoethanolamine) and MEA/TEA (monoethanolamine/triethanolamine). Coupons on the bottom were immersed in fluids that also contained 3A40 (3-amino-4-octanol). (Figure courtesy of ANGUS Chemical Co.)
Differentiate performance
Figure 4. Phosphate free MWF formulations containing a triazine biocide were evaluated at a pH of 10 in coupon testing at a 5% dilution in 200 ppm water hardness. Fluids were tested in the following amines: AMP (2-amino-2-methyl-1-propanol), AB/AEPD (2-amino-1-butanol/2-amino-2-ethylpropanediol), DGA (diglycolamine), MIPA (monoisopropanolamine), MEA (monoethanolamine) and MEA/TEA (monoethanolamine/triethanolamine). Coupons on the bottom were immersed in fluids that also contained 3A40 (3-amino-4-octanol). (Figure courtesy of ANGUS Chemical Co.)
Differentiate performance
Minch believes the best way to differentiate the performance of an aluminum stain inhibitor is to work with a model fluid formulation. He says, “Inhibitors should be used at the same percent active in the model formulation, and the level of staining observed should be compared on immersed coupons. The formulation that provides the least amount of staining (assuming no weight loss occurred) without negatively impacting other fluid performance parameters is the best inhibitor. Again, trying to match conditions and metallurgy to the actual expected field conditions are the key to success.”
Cole recommends two strategies for differentiating the performance of individual aluminum stain inhibitors. She says, “Performance of potential inhibitors should be compared against two controls (deionized water and hard water) as well as a formulated fluid that does not contain added corrosion protection (just triethanolamine [TEA] and monoethanolamine [MEA]). Consideration should be given to examining galvanic corrosion by potentially managing the cast iron chip test and separating the metals afterwards to see if any weight loss occurred on the aluminum chips.”
Formulator’s perspective
Two representatives from MWF formulators were asked for their perspective on aluminum stain inhibitors and whether the current options are satisfactory or is there need for better alternatives.
STLE-member Jennifer Lunn, senior chemist at JTM Products Inc. in Solon, Ohio, says, “There are several great options for aluminum stain inhibitors that exist in the market today. These options even play a dual role when formulating; they provide not only protection of the aluminum, but also contribute to the extreme pressure performance of the fluid, lowering formulation complexity. Until phosphate ester chemistry is regulated in MWFs, these really are a great choice when needed.”
STLE-member Kevin Saunderson, director of technology at New Age Chemical in Delafield, Wis., says, “Overall, I have found the currently available additives, when used in water-based MWFs, effective in protecting a majority of the aluminum alloys used by our customers from staining. The number of available options, their relative ease of use and reasonable cost have made this performance aspect easily attainable.”
When asked about amines, Saunderson indicates that they are a necessary evil in formulating water-based MWFs and machining aluminum alloys. He says, “However, the amines commonly chosen by MWF formulators offer far too many benefits to be ignored and can still be used effectively on a wide variety of aluminum alloys in use today. The challenge involves properly balancing the desire for excess alkalinity (buffering capacity) which can benefit product longevity, with the desire for aluminum compatibility. While all amines contribute to aluminum staining to a certain degree, how the amine is neutralized is almost as critical as the choice of amine. Proper selection of the acid-functional component can significantly improve compatibility of the in-use fluid with aluminum while potentially reducing the need for costly inhibitors.”
Lunn says, “The tried and true amines work and are cost effective in their traditional functions. But supplementary additives often need to be added to counteract the negative impact they might have on performance, such as aluminum staining. Some of the new amine chemistries available to the market currently have shown the ability to reduce aluminum staining and also have a dual purpose which can assist with lowering formulation complexity.”
Saunderson welcomes better amine options to deal with aluminum staining. He says, “The current amines have been and will continue to be used successfully by MWF formulators. However, there will always be interest in new or better whether it involves a new molecule or a new synergy with an existing amine.”
Lunn recognizes that the increasing use of aluminum in applications such as automobiles will mean that “aluminum friendly” amines and aluminum stain inhibitors will continue to be important additives for the MWF formulator. She says, “As more aluminum is incorporated into a vehicle’s makeup, metalworking fluids will need to be adapted to account for their growing use.”
Saunderson feels that alternative chemistries for aluminum stain inhibition already exist. He says, “The challenge could involve rebalancing existing formulas to accommodate the change in additive chemistry followed by extensive testing to validate equal or better performance. Depending on the number of formulas and the overall scope of the change, this could take significant time. With the uncertainty around the potential regulatory timeline, it makes sense to have a plan in place sooner rather than later.”
There is concern that the use of phosphorus compounds may now be restricted in MWFs because they are considered as the root cause for algae bloom formation. This may lead to increasing challenges for formulators in minimizing aluminum staining (
3). Lunn says, “With the increased accumulation of phosphates in the environment, it is possible they will come under scrutiny in metalworking fluids. If that happens, specialty amines will play a very important role in formulating ‘aluminum friendly’ fluids.”
Future trends
Chen predicts there will be greater demand for aluminum stain inhibitors. He adds, “Inhibitors of higher effectiveness are needed with a preference for organic based inhibitors.”
Potnis believes there will be more focus on aluminum stain prevention in formulations via multifunctional additives rather than specific inhibitors. He says, “This market is developing a greater understanding of aluminum staining and how specific chemistries, such as phosphate esters and acid-amine salts, can create concerns as well as innovative solutions. For example, utilizing chemistries such as 3A4O and AB/AEPD to address multiple performance attributes (e.g., fluid longevity, aluminum staining, cast iron corrosion, pH stability, etc.) allows potential regulatory and cost savings benefits on top of performance.” How the amine is neutralized is almost as critical as the choice of amine.
Minch focuses on the increasing use of aluminum in the automotive industry. He says, “One of the more certain trends regarding aluminum stain inhibitors is their expected growth relative to increasing aluminum consumption in some markets. Automotive is one major market where aluminum stain inhibitors may see significant growth. Steel and aluminum comprise the majority of materials within today’s automobiles and are expected to continue that leadership position for years to come. One of the main drivers for aluminum usage is its lightweight properties, which has been one major pathway for OEMs to achieve higher government-mandated mileage and emission standards. In fact, SME recently noted that 200,000 tons of aluminum capacity will ‘come on line’ early next year for the automotive market; the implication is that this volume will be used for structural and body components (
4).”
With the automotive industry forecasting that electric vehicles (EVs) will replace internal combustion engine automobiles over time, Minch predicts that lightweighting will continue to be a concern for the EV market. He says, “Aluminum is expected to be a solution for these vehicles as well. Aluminum Insider expects that EV aluminum demand will be near 825,000 mt in 2019 and may grow ten-fold by 2030. Applications for this aluminum usage include sheet, extrusion, battery components and even EV charging stations (
5). Mitigating corrosion in EVs is especially of concern, both of which must be protected to ensure good performance (
6).
Minch concludes by stating that the trend to diversify materials used to produce automobiles beyond just steel and aluminum will lead to a growing need for more effective corrosion/stain inhibitors. He says, “Such diversification of materials can potentially cause galvanic corrosion issues where metals such as aluminum reside. As long as this is practiced, corrosion inhibition practices will need to be fully investigated to ensure that vehicles meet their lengthy corrosion performance requirements (
6).”
Cole believes the need for better corrosion protection in general will lead to the development of packages that will inhibit aluminum and ferrous corrosion. She also indicates that many of these products will exhibit multifunctional characteristics.
Another trend to facilitate the use of aluminum in automotive applications is known as vacuum impregnation. Cole says, “This technique is not necessarily an inhibition technology but rather an approach to facilitate the movement of molten aluminum via vacuum delivery into a mold to ‘impregnate’ the voids and pores in a cast part by eliminating points of entry from potential penetration from electrolytes.”
Harwood says, “Demand for aluminum stain inhibitors is growing because of the use of certain aluminum grades (such as the 7000 series) that are being more widely used in the aerospace and automotive sectors. These grades are more susceptible to corrosion/staining.”
Inhibitors for preventing staining of aluminum alloys have become a very important additive for the metalworking fluid formulator. Proper selection of these inhibitors in combination with amines will be necessary now and in the future as a more diverse number of materials will be used in such applications as automobiles.
REFERENCES
1. Click
here.
2. Click
here.
3. Click
here.
4. Click
here.
5. Click
here.
6. Click
here.
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. You can reach him at neilcanter@comcast.net.