•
A three-dimensional cryptand-like triazolo cage has a strong affinity for trapping chloride anions.
•
The triazolo cage demonstrated a preference for binding chloride anions compared to other anions.
•
Immersion of a steel sample coated with the triazolo cage in a saturated sodium chloride solution led to no evidence for corrosion after two weeks at ambient temperature.
Pure water suitable for drinking and industrial processes is becoming more difficult to obtain. Techniques such as desalination are becoming commercially available but are expensive because they are energy intensive.
Readily available seawater is more widely used, which makes it imperative to find a more cost-effective approach for removing chloride anions. Inhibition of chloride corrosion on steel alloys continues to be a significant challenge that can impact the use of specific lubricants such as water-dilutable MWFs.
From an inhibition standpoint, the key to protecting metal surfaces is to find a candidate that can chemically bind chloride anions preferentially over all other electrolytes. In a previous article, an aryl-triazole foldamer was developed that preferentially binds chloride anions (
1). The process is reversible under the influence of ultraviolet light, which converts the foldamer from a helix to a random orientation and back again with visible light.
But the chloride anion binder was only effective in an organic solvent and not in an aqueous environment. Amar Flood, the James F. Jackson Professor of Chemistry and Luther Dana Waterman Professor in the department of chemistry at Indiana University in Bloomington, Ind., was involved in preparing and evaluating the foldamer. He and his research group have continued to work to find a more effective chloride anion binder.
Flood says, “Two of the challenges in finding a binder is to identify molecular binding units that stabilize chloride anions and convert them into receptors that exhibit selectivity. The key is to synthesize a receptor with a good size and shape match for the chloride anion.”
One approach to achieve this goal is to utilize hydrogen bonding as a means to bind chloride anions. Traditionally, hydrogen bonding was believed to be only effective when the hydrogen atom was chemically bound to a highly electronegative heteroatom such as oxygen or nitrogen. Says Flood: “Big electronegativity differences between hydrogen and an atom such as oxygen lead to hydrogen bonding. A recent review has determined that the intrinsic electronegativity difference present between carbon and hydrogen is sufficient enough to support hydrogen bonding.”
Placement of a specific carbon-hydrogen bond within the framework of a specific molecule can significantly amplify the strength of the hydrogen bonding. Flood says, “We have had success working with 1,2,3-triazole derivatives. A carbon-hydrogen bond adjacent to a triazole functionality is 85% as effective in hydrogen bonding as a nitrogen-hydrogen bond contained within a pyrrole derivative.”
Flood and his colleagues have now prepared a new triazole derivative that demonstrates excellent affinity for binding chloride anions.
Cryptand molecule
The molecule synthesized by the researchers is a three-dimensional “cage” that consists of six triazole “motifs” that is perfectly shaped to trap chloride anions. An image of the molecule is shown in Figure 3 (chloride anion is shown in green). Flood says, “We characterize this molecule as a cryptand, which is an inherently three-dimensional molecule that can attract and capture a specific chemical species so effectively that it will not come out.”
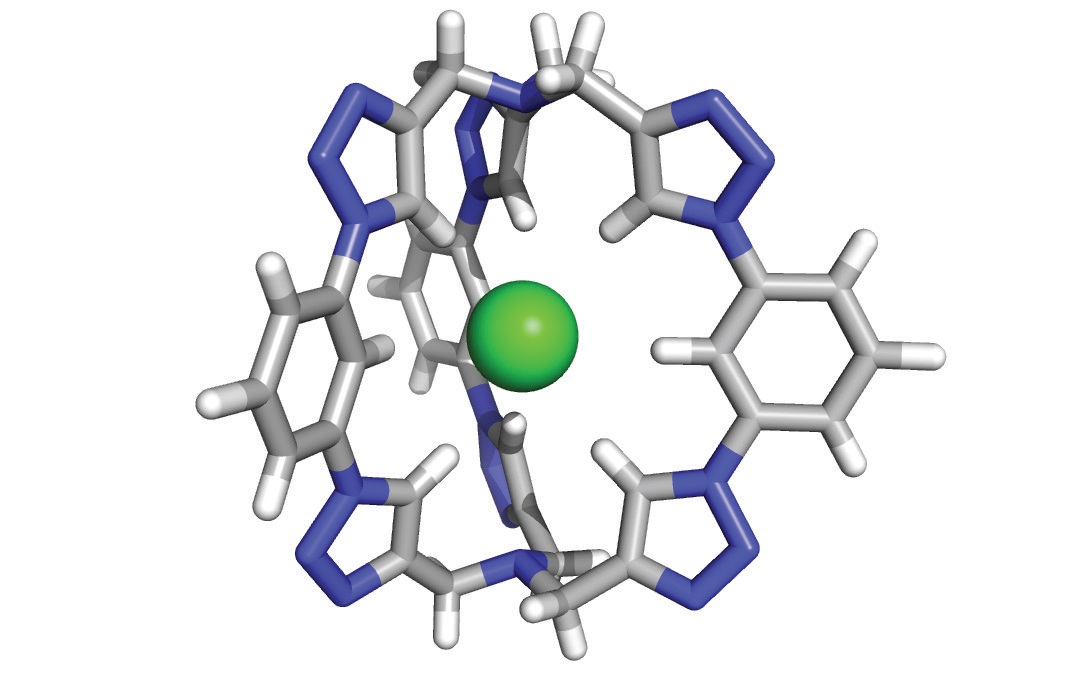 Figure 3. A new three-dimensional molecule shaped in the pattern of a cage has been found to very effectively trap a chloride anion (shown in green). (Figure courtesy of Indiana University.)
Figure 3. A new three-dimensional molecule shaped in the pattern of a cage has been found to very effectively trap a chloride anion (shown in green). (Figure courtesy of Indiana University.)
The cryptand-like triazolo cage was synthesized in a one-step process involving the reaction of a C
2-symmetric bisazide with a C
3-tripropargylamine in a copper (I) catalyzed azide alkyne cycloaddition reaction. An initial clue to the chloride anion affinity of this molecule came during the purification process used to isolate the triazolo cage. The researchers found that a sodium chloride complex of the triazolo cage was isolated during column chromatography even though none of this salt was deliberately added.
Affinity for chloride anions was determined through the use of competitive titrations followed by evaluation of shifts in CH donor peaks in 1H nuclear magnetic resonance spectroscopy. Initial work was done in the polar solvent, dimethylsulfoxide (DMSO).
When evaluating other anions, the researchers found that the triazolo cage exhibited selective binding of chloride anions. For example, binding of chloride anions was one million times more effective than binding of iodide anions.
The triazolo cage also was able to extract chloride anions from an aqueous medium into the solvent, dichloromethane. Flood says, “The biggest challenge for any chloride binder is to extract it from water. While organic solvents are not competitive, water displays a high dielectric constant and high hydration energy making it difficult to remove chloride anions.”
The researchers found that the triazolo cage was able to extract chloride anions from aqueous solutions of ammonium chlorides and alkali metal chlorides such as sodium.
The rigidity of the triazolo cage was a very important structural characteristic in the molecule’s ability to bind chloride anions. Flood says, “We developed a more flexible version of the triazolo cage and found that this untethered form of the cage did not demonstrate the binding affinity of the more rigid version.”
The researchers evaluated the corrosion inhibition characteristics of the triazolo cage by coating a mild steel sample that was immersed in a saturated sodium chloride solution for two weeks at ambient temperature. The coating remained on the steel sample and there was no evidence seen of any corrosion.
Future work for the researchers will focus on determining the mechanism for how the triazolo cage binds and can then release chloride anions. Additional information can be found in a recent article (
2) or by contacting Flood at
aflood@indiana.edu.
REFERENCES
1.
Canter, N. (2011), “Chloride binder,” TLT,”
67 (1), pp. 12-13.
2.
Liu, Y., Zhan, W., Chen, C. and Flood, A. (2019), “Chloride capture using a C-H hydrogen-bonding cage,”
Science,
365 (6449), pp. 159-161.