Superhydrides that contain a large number of covalently bonded hydrogens around a central metal atom are considered to be the leading candidates to exhibit superconductivity at room temperature.
A new lanthanide superhydride has been synthesized that shows superconductivity approximately 20 K below room temperature.
The mechanism for how the lanthanide superhydride develops superconductivity is not known.
Preparation of superconductive materials remains a long-term research goal. The appeal of working with superconductive materials is that they can conduct electricity without creating any resistance or friction. This means that none of the energy is dissipated as heat.
Maddury Somayazulu, research associate professor at the school of engineering and applied sciences at George Washington University in Washington, D.C., says, “The ultimate goal or Holy Grail is to identify a substance that will exhibit superconductivity at room temperature. Since the prediction of metallization of hydrogen at high pressures and the subsequent prediction by professor Neil Ashcroft that hydrogen would simultaneously be metallic and superconducting at room temperature, there has been sustained research toward metallizing hydrogen. Simultaneously, due to the prediction that molecular hydrogen in the right environment also would be a superconductor albeit at high pressures, the search has enlarged to include hydrides and hydrogen-bearing systems. With the discovery of superconductivity in hydrogen sulfide (H
3S—which was predicated by theoretical predictions based on the synthesis of a high-pressure compound—[H
2S]
2H
2), the race was on to synthesize and investigate potential superconductivity in other systems such as yttrium hydride, lanthanum hydride and calcium hydride.”
In a previous TLT article, work conducted to synthesize superconductive polyhydrides for the first time was described (
1). The experimental conditions used were temperatures reaching 2,000 K and pressures between 30-40 gigapascals. X-ray analysis indicated that the species formed was a sodium polyhydride with a NaH
3 stoichiometry. A second species identified had seven hydrogen atoms surrounding a sodium atom. Based on analytical data, the researchers speculated that three atom hydrogen chains and two atom hydrogen molecules were both present in this polyhydride.
Somayazulu feels that a large number of covalently bonded hydrogens are needed to surround a central metal atom. He says, “It is desirable to establish a framework of hydrogen molecules with stabilized bond lengths that are greater than a normal hydrogen molecule. Under pressure, the covalent bonds become metallic and on further increasing the pressure, these materials known as superhydrides acquire a unique configuration wherein the electron-phonon coupling is enhanced and superconductivity is attained.”
To achieve this result, hydrides are the simplest molecules to work with to develop superconductivity. Somayazulu says, “Most known hydrides contain two to three hydrogen atoms per metal atom and therefore result in an insufficient density of hydrogens to drive the transition temperature higher.”
A significant development was finding that hydrogen sulfide was converted under moderate pressures to a novel sulfur hydrogen compound. Somayazulu says, “Theoretical calculations determined that application of high pressures would produce a hydrogen sulfide derivative that exhibits superconductivity. Empirical results showed that hydrogen sulfide and hydrogen mixed to create a cubic crystal with the empirical formula of H
3S that displays superconductivity in the 200 K temperature range.”
Theoretical analysis shows that increasing the hydrogen content of a compound should increase the temperature where superconductivity is observed. Somayazulu says, “We believe that compounds with multiple hydrides can be formed where there are no bonds seen between the hydrogen atoms and a central metal atom. As pressure is increased, atomic orbitals are forced to overlap creating the right conditions for peak electron-phonon coupling.”
Somayazulu and his colleagues have now evaluated compounds with higher hydrogen content where the central metal atom is surrounded by more than six hydrogen atoms. These substances are known as superhydrides.
Pulsed laser heating
The researchers worked with the rare Earth metal, lanthanide, and ammonia borane was used as the hydrogen source. Both materials were placed in a diamond-anvil cell and subjected to severe temperatures and pressures using pulsed laser heating.
Upon heating at a temperature between 1,000-1,500 K under pressures above 175 gigapascals, X-ray diffraction analysis shows that the resulting lanthanide superhydrides formed a face-centered cubic structure with a structure of LaH
10 (
see the left structure in Figure 2). During the synthesis, the samples were kept at a temperature below 1,800 K by varying the combination of laser power and pulse width of the heating laser since higher temperatures resulted in the formation of other borohydrides, which are not superconducting.
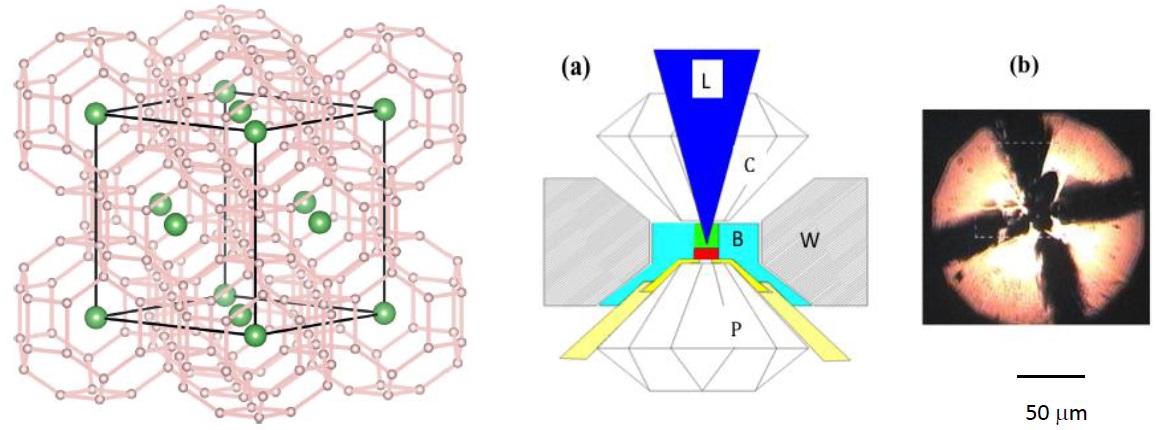 Figure 2. A lanthanide superhydride with a face-centered cubic structure shown in the left structure was synthesized using the diamond-anvil cell shown in 2a. An optical micrograph of this superhydride is shown in 2b. (Figure courtesy of George Washington University.)
Figure 2. A lanthanide superhydride with a face-centered cubic structure shown in the left structure was synthesized using the diamond-anvil cell shown in 2a. An optical micrograph of this superhydride is shown in 2b. (Figure courtesy of George Washington University.)
The structure of the lanthanide superhydrides created in this manner was determined by X-ray diffraction. Figure 2a shows an illustration of the diamond-anvil cell where L represents the laser heating, c is the cylinder diamond, W is the tungsten outer gasket and P is the piston diamond.
Figure 2b is an optical micrograph of a lanthanide superhydride sample formed under a pressure of 178 gigapascals.
The researchers conducted electrical resistance measurements to determine the temperature at which superconductivity occurred. When samples of the lanthanide superhydrides were subjected to cooling, a decrease in electrical resistance was initially found at 275 K, which is approximately 20 K below room temperature. A more significant reduction was detected at 260 K.
Upon reheating the sample, the resistance increased at a lower temperature, 245 K, which is not consistent with the observation seen during the cool down. Somayazulu says, “We believe that this shift in temperature occurred because the ordering or disordering of hydrogen atoms under our experimental conditions is not consistent. Every single hydride does not display the same behavior unless we maintain the same P-T paths.”
This research demonstrates the first evidence of a material exhibiting superconductivity near room temperature. Somayazulu says, “We do not yet understand what is the exact microscopic mechanism that results in the superconductivity and experiments are underway to unravel this. Our biggest challenge is to establish the Meissner effect where an applied magnetic field results in changes in the electrical resistivity. These are difficult experiments and we are trying to establish this effect on our samples.”
Future work will involve preparation of more complex superhydride compounds that contain even higher levels of hydrogen per lanthanide atom. Additional information can be found in a recent article (
2) or by contacting Somayazulu at
zulu58@email.gwu.edu.
REFERENCES
1.
Canter, N. (2016), “Sodium polyhydrides: Potential superconductors,” TLT,
72 (11), pp. 10-11.
2.
Somayazulu, M., Ahart, M., Mishra, A., Geballe, Z., Baldini, M., Meng, Y., Struzhkin, V. and Hemley, R. (2019), “Evidence for Superconductivity above 260 K in Lanthanum Superhydride at Megabar Pressures,”
Physical Review Letters,
122 (2), 027001.