KEY CONCEPTS
•
Bacteria can produce hydrogen in the absence of light through a process known as dark fermentation.
•
A genetic mutation of the anaerobic bacterium
Thermotoga maritima enables the production of up to eight moles of hydrogen per mole of glucose, which increases the yield about the theoretical limit.
•
The reason for the dramatic increase in hydrogen production is the bacterium is using not just fermentation but also a second process known as the pentose phosphate pathway.
Alternative energy propulsion system development is accelerating leading researchers to look for strategies to produce needed raw materials essential for these processes. A case in point is the growing need for hydrogen and hydrogen infrastructure to support the expanding production of fuel cells vehicles from OEMs such as Toyota.
While synthetic approaches have been actively pursued, researchers also are looking to use microbes such as bacteria to manufacture fuels through biochemical pathways. The objective is to produce these fuels in an efficient and sustainable manner.
In a previous TLT article, this approach was used by researchers to produce ethanol from a mixture of carbon monoxide hydrogen and carbon dioxide using a process called syngas fermentation (
1). In achieving the synthesis of ethanol, researchers were able to better understand how the specific bacterium,
Clostridium ljungdahlii can be prompted to produce ethanol instead of using a more favored pathway to produce biomass.
Bacteria also can produce hydrogen through a process known as dark fermentation. Paul Blum, Charles Bessey Professor of biological sciences at the University of Nebraska in Lincoln, Neb., says, “Microorganisms can conduct fermentation in a number of different ways. Dark fermentation is carried out by microbes in the absence of light. In many cases, the microbes are anaerobic bacteria, which do not tolerate the presence of oxygen. Fermentation also is seen in algae that obtain much of their energy through photosynthesis.”
Blum indicates there are multiple ways for microorganisms to produce hydrogen, but in most cases the reactions are not prioritized, in part because hydrogen can be toxic. He says, “Hydrogen formation occurs due to the need for the microorganisms to balance oxidation and reduction reactions. For example, the formation of ethanol is an oxidation reaction that must be balanced through reduction.”
One obstacle to using dark fermentation is that hydrogen production is limited theoretically to a maximum of four moles of hydrogen gas per mole of the starting material, glucose. Blum says, “In evaluating options to exceed this theoretical limit, we became interested in working with the anaerobic bacterium
Thermotoga maritima, which is unique compared to all other bacteria and classified in its own Phylum.”
Thermotoga maritima thrives under extreme conditions present in hot springs as well as hydrothermal deep-sea vents. It is known as a hyperthermophile because of its desire to grow under high temperature conditions (80 C).
These conditions also are quite favorable for hydrogen formation as compared to ambient temperature. Blum and his colleagues, knowing that
Thermotoga maritima produces hydrogen at the theoretical yield, worked to determine a way to manufacture hydrogen above the theoretical limit.
Genetic mutation
The researchers used genetic mutation to enable a new version of
Thermotoga maritima to produce up to eight moles of hydrogen per mole of glucose (
see Figure 3). This represents the synthesis of 46% more hydrogen per cell than
Thermotoga maritima and dramatically increases the yield above the theoretical limit.
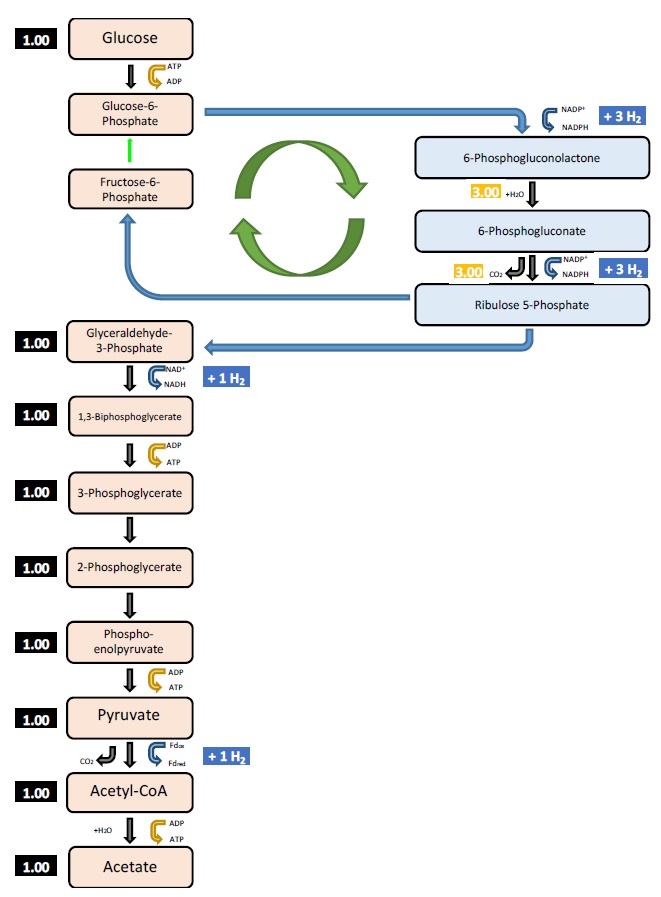 Figure 3. A new version of the anaerobic bacterium Thermotoga maritima has produced a much higher yield of hydrogen by using the glycolysis and pentose phosphate pathways shown here. (Figure courtesy of The University of Nebraska-Lincoln.)
Figure 3. A new version of the anaerobic bacterium Thermotoga maritima has produced a much higher yield of hydrogen by using the glycolysis and pentose phosphate pathways shown here. (Figure courtesy of The University of Nebraska-Lincoln.)
Says Blum: “Increasing hydrogen production through fermentation has been a Holy Grail for the field for the past 30 years. We achieved this result by inactivating a gene in the bacterium that slowed hydrogen production. The result led to the mutation of a second gene that was responsible for transporting sugar in the bacterium. The mutation was needed to minimize the formation of high concentrations of sugars that are lethal to the bacterium. But this mutation also prompted
Thermotoga maritima to switch from biomass to hydrogen formation.”
The key reason for the result is that the bacterium not only uses glycolysis to generate hydrogen but also takes advantage of a parallel process known as the pentose phosphate pathway (PPP). Hydrogen was produced by placing various strains of the bacterium in small batch cultures and evaluating the results after a single growth cycle that lasted for 20 hours at 80 C.
Figure 3 shows how the glycolysis and pentose phosphate pathways are interconnected leading to the generation of the much higher percentage of hydrogen per cell.
The next step is to determine how this process can be scaled up. Blum says, “Attempting to produce hydrogen
in-vitro can work but will be too expensive to commercialize. The best opportunity is to utilize a continuous fermentation culture containing the bacterium.”
Blum believes there is a good deal of feedstock flexibility because
Thermotoga maritima will work with any of 14 sugar hexoses. In the current study, the researchers worked with maltose, which was readily available to them.
In operating a continuous fermentation culture, the only things that need to be done will be adding new nutrients and efficiently removing and isolating the hydrogen generated. A new paper is under review demonstrating this accomplishment at a larger scale that includes process parameters. The temperature will need to be maintained at 80 C, but no agitation is required since that would add oxygen to a system where an anaerobic environment is required.
Blum says, “This technique can be used not only to produce hydrogen but potentially other metabolites. We have also used
Thermotoga maritima to prepare a microbial cell battery that also uses low cost sugars as nutrients to produce power.”
Additional information on this research can be found in a recent article (
2) or by contacting Blum at
pblum1@unl.edu.
REFERENCES
1.
Canter, N. (2017), “Biofuel production using syngas fermentation,” TLT,
73 (1), pp. 14-15.
2.
Singh, R., White, D., Demirel, Y., Kelly, R., Noll, K. and Blum, P. (2018), “Uncoupling Fermentative Synthesis of Molecular Hydrogen from Biomass Formation in Thermotoga maritima,”
Applied and Environmental Microbiology, DOI: 10.1128/AEM.00998-18.