KEY CONCEPTS
•
A new nanoforming process known as roll-to-roll laser-induced superplasticity has been developed.
•
Use of a carbon dioxide laser induces the generation of a shockwave that leads a superplastic flow of metal into the nanomold.
•
Superplasticity minimizes friction and expedites the release of the patterned metal from the nanomold while resulting in low tool wear and heat generation.
The reduction in the size of metal parts and the increase in the intricacy of these parts is leading to the need for improved metalworking processes that can meet demanding specifications and still be cost effective. This is particularly important at the nanoscale where machinery such as microelectromechanical systems is required.
In a previous TLT article, photochemical machining (PCM) was discussed as a method to produce precise metal parts (
1). PCM is used to machine parts with thicknesses less than one millimeter, including nanoparts. In PCM, the metal substrate is exposed to ultraviolet light to form the specific parts image. A photo-resistant coating is applied to prevent certain areas of the substrate from being exposed to ultraviolet light.
Ramses Martinez, assistant professor of Industrial Engineering and Biomedical Engineering at Purdue University in West Lafayette, Ind., says, “There are many processes available to conduct machining at the nanoscale. Molding is most commonly used, but the resolution for this process is 800 nanometers, which is not satisfactory. In the manufacture of parts such as high-resolution transistors, ultraviolet light is used to improve resolution, which is very expensive.”
Metal forming at the nanoscale (known as nanoforming) is particularly challenging. Martinez indicates that a conventional process where the substrate is heated up to increase fluidity and then pushed into a mold does not work at the nanoscale because the pattern of the die is smaller than the grain size of the metal. Another concern is that operations after the metal forming such as metallization and lift off can lead to increased processing time, complexity and, as a consequence, cost.
A new approach for nanoforming is needed that can increase the resolution to between 50 and 80 nanometers. Such a process has now been developed.
Superplasticity
Martinez and his colleagues developed a new nanoforming process known as roll-to-roll laser-induced superplasticity (R2RLIS) that is a cost-effective metal-forming process that can be conducted at the nanoscale. He says, “This process is induced by the use of a carbon dioxide laser that facilitates the flow of the metal into a nanomold. This step is followed by nanowelding the metal to a flexible, plastic substrate, prepared from polyethylene terephthalate. Release from the nanomold then occurs to create the part.” A schematic of R2RLIS is shown in Figure 1.
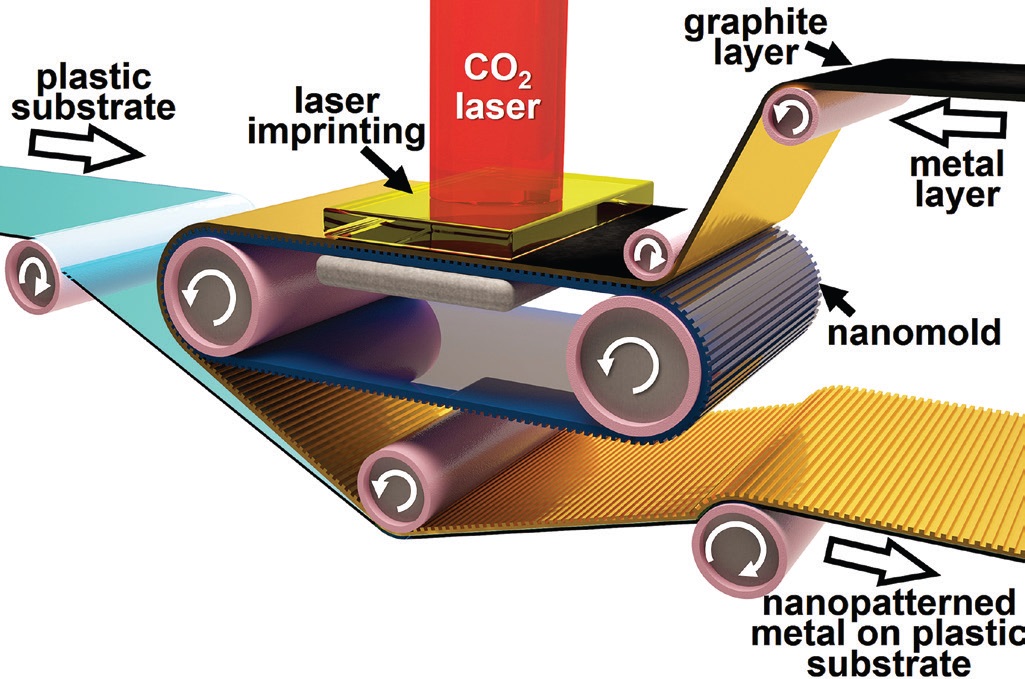 Figure 1. Metal forming now can be accomplished more efficiently at the nanoscale using a technique called roll-to-roll laser-induced superplasticity. (Figure courtesy of Purdue University.)
Figure 1. Metal forming now can be accomplished more efficiently at the nanoscale using a technique called roll-to-roll laser-induced superplasticity. (Figure courtesy of Purdue University.)
Initially a set of rubber rolls applies a metallic film coated with graphite onto one of the sides of a flexible, plastic substrate under a confinement layer that is a transparent zinc selenide or calcium fluoride window. The carbon dioxide laser then introduces an intense amount of energy leading to the removal of the five-micron thick graphite layer, which generates a shockwave for approximately one millisecond. As the metal substrate is confined, the energy leads to the superplastic flow of the metal into an epoxy-based nanomold coated with a thin layer of titanium.
Martinez says, “The use of graphite, a solid lubricant, facilitates the patterning of metals at the nanoscale, since the shockwaves generated when the graphite is irradiated with the laser force the metal substrate to flow into the nanomold.”
Inducing superplasticity into metal reduces the friction significantly along the whole nanoforming process. Martinez says, “The low friction between the metal and the nanomold minimizes heat generation and tool wear, facilitating the release of the patterned metal from the nanomold.”
The researchers evaluated this technique using gold, silver and the high-conductivity metals aluminum and copper. Martinez says, “Forming silver has been very challenging in the past because this metal oxidizes easily under typical forming conditions. The low heat generated during R2RLIS minimizes the oxidation of the metals used, facilitating the nanoforming of silver.”
The process has improved the resolution of the parts down to 40 nanometers. Martinez says, “We have been able to achieve this high resolution under ambient conditions without the need of a clean room environment.”
The nanoforming process reduces the size of the metal grains leading to an increase in hardness. Martinez says, “By adjusting the power density of the carbon dioxide laser, the final mechanical properties of the metal used in the nanoforming process can be tuned for specific applications. For example, we demonstrated a 30% increase in the hardness of the gold films used from 1.96 gigapascals before the process to 2.55 gigapascals.”
Future work will involve determining if the carbon dioxide laser can be replaced with a less expensive laser. Martinez says, “Our objective is to make this nanoforming process less costly and more readily available by using a less-expensive blue laser diode that displays a visible beam at a wavelength of 600 nanometers.”
Additional information can be found in a recent article (
2) or by contacting Martinez at
rmartinez@purdue.edu.
REFERENCES
1.
Canter, N. (2013), “Photochemical machining,” TLT,
69 (3), pp. 12-13.
2.
Goswami, D., Munera, J., Pal, A., Sadri, B., Scarpetti, C. and Martinez, R. (2018), “Roll-to-Roll Nanoforming of Metals Using Laser-Induced Superplasticity,”
Nano Letters,
18 (6), pp. 3616-3622.
.