KEY CONCEPTS
•
Block polymers with at least two covalently bonded components are under development for use as improved polymer membranes in water treatment.
•
These polymers are self-assembled into nanostructures with pores that are well defined and exhibit the same size.
•
High through-put and high selectivity can be achieved by tailoring the block polymer with different functional groups depending upon the application.
Water is becoming a more valuable resource as its availability becomes more limited. This statement applies not just to consumers but also to industrial applications. For example, end-users of metalworking fluids consume large quantities of water and need to carefully waste treat them to comply with local effluent regulations.
The increasing use of hydraulic fracturing as a means to produce oil and natural gas also has led to greater consumption of water. In a previous TLT article, a superhydrophilic membrane based on aluminum oxide has been found to effectively remove contaminants present in water used for hydraulic fracturing (
1). The researchers were able to remove more than 90% of the organic contaminants in water without limiting the flow rate through the membrane.
Commercially available polymer membranes are widely used to filter water but have significant limitations. William Phillip, associate professor in the department of chemical and biomolecular engineering at the University of Notre Dame in South Bend, Ind., says, “Currently used membranes have nanostructures without a single, well-defined pore size. These are needed to more efficiently remove contaminants from water during the cross-flow separation process. A second concern is that a trade-off exists between membrane through-put and selectivity. Membranes that demonstrate high through-put display low selectivity, while those membranes showing high selectivity exhibit low through-put.”
Another problem encountered during the operation of membranes is fouling, which means that pores become plugged up hindering and/or preventing water flow. In metalworking fluids systems, problems have been encountered in the past with defoamers, although this has improved recently.
Phillip says, “In fouling, a variety of molecules can stick to the polymer membrane. One established approach for minimizing this issue is to tailor the surface chemistry of the membrane to resist adhesion of the contaminants present in a particular source of water. Current polymer membrane technology struggles because the fouling characteristic vary from source to source and no single surface chemistry is a panacea for reducing fouling.”
Polymer membranes are needed in two applications for purifying water. Phillip says, “Desalination is becoming a priority with salt water readily available. The challenge is that the high osmotic pressures of saline sources makes desalination energy intensive. This has driven interest in hybrid membranes that seek to treat water to the purity levels required by its end-users, for example, by removing hard divalent cations to prevent scaling or to recover useful resources such as nitrates and phosphates.”
A new membrane technology is now under development that has the potential to overcome some of the limitations encountered with existing polymer membranes.
Block polymer membranes
Phillip indicated that research to improve polymer membranes is moving to the use of block polymers that consist of at least two covalently bonded components. He says, “An attractive feature of block polymers is that they can self-assemble into membrane nanostructures with pores that are typically well defined and exhibit the same size. This enables block polymer membranes to display both high through-put and high selectivity.”
The technique used to prepare block polymer membranes is known as self-assembly and non-solvent induced phase separation (SNIPS). Phillip says, “This preparation method combines the self-assembly characteristic of block polymers with the known way to prepare commercially available membranes. The polymer used is dissolved in a suitable solvent, cast into a thin film on a substrate and then plunged into a non-solvent. The liquid-liquid separation enables the block polymer to self-assemble.”
Phillip furnished an example of a three-component block polymer that his research group is evaluating in collaboration with Purdue University. He says, “We combine polystyrene, which is rigid but also can be brittle, with polyisoprene to contribute pliability and elasticity similar to rubber. These two polymers generate a hydrophobic matrix. The third polymer is poly (dimethylacrylamide) and is used to line the pore walls. Poly (dimethylacrylamide) has reactive groups enabling researchers to modify the pore walls with a variety of different functional groups depending upon the application. For example, mercaptan-based binding ligands can be used to capture heavy metals.”
Phillip also indicated that groups with anionic and cationic charges can be added to functionalize block polymer membranes as a way to remove calcium and magnesium cations to soften the water.
Three general approaches for incorporating functional groups within the membrane pores can be broadly categorized as direct incorporation, graft after casting and coupling reactions. This flexibility is appealing because it enables block polymer membranes to be used in charge separation, selective adsorption, anti-fouling and chemical conversion applications (
see Figure 3). Phillip says, “All of these techniques work well but can take a long time to be executed, which is an impediment to commercialization. We prefer using coupling reactions to modify block polymer membranes because they can be completed quickly.”
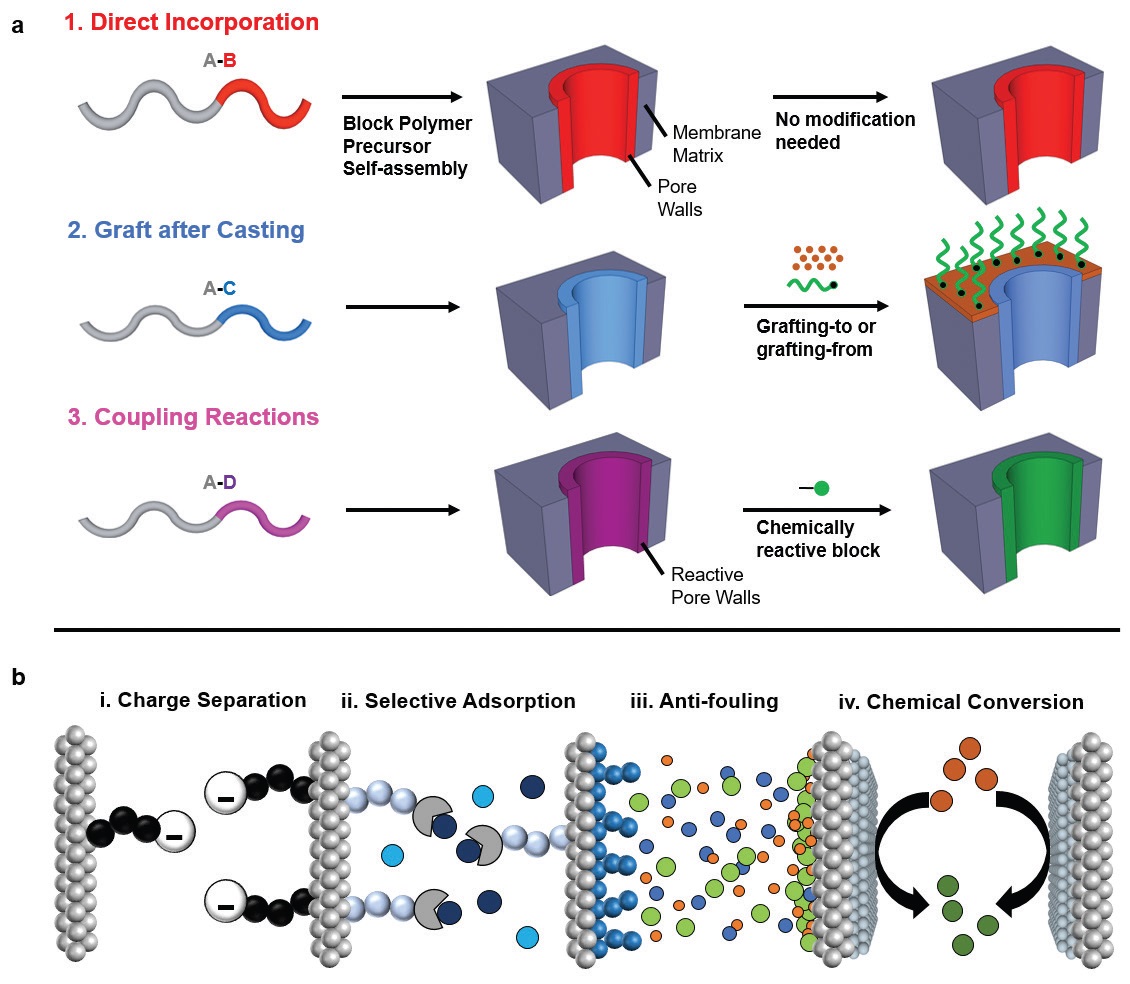 Figure 3. Block polymers have become an appealing material for polymer membranes for use in water treatment because they can be functionalized by using three different methods enabling them to be used in four distinct applications. (Figure courtesy of the University of Notre Dame.)
Figure 3. Block polymers have become an appealing material for polymer membranes for use in water treatment because they can be functionalized by using three different methods enabling them to be used in four distinct applications. (Figure courtesy of the University of Notre Dame.)
Phillip believes that there are two areas where block polymer membrane technology can be improved. He says, “We are working to determine how to turn these membranes into multifunctional devices by creating a way for them to change color or charge when exposed to a specific contaminant such as a heavy metal. A second objective is to determine how to design membranes that selectively permeate dilute, target solutes during the filtration process instead of concentrating the solutes in the retained solution, which requires large areas of membrane.”
Additional information on block polymer membranes can be found in a recent review (
2) or by contacting Phillip at
wphillip@nd.edu.
REFERENCES
1.
Canter, N. (2018), “Waste treatment of fracking water,” TLT,
74 (1), pp. 16-18.
2.
Zhang, Y., Arbelo, N., Weidman, J., Corti, D., Boudouris, B. and Phillip, W. (2018), “Fit-for-purpose block polymer membranes molecularly engineered for water treatment,”
npj Clean Water,
1, Article #2.