Tuning atomic topographies by tribochemistry
Drs. Wilfred T. Tysoe & Nicholas D. Spencer | TLT Cutting Edge August 2018
Tribochemical reactions allow precise control over the depth of etched features on hydroxylated silicon—down to a single atomic layer!
We have recently discussed the way in which the tribochemical reaction rates of a lubricant additive, zinc dialkyldithiophosphate, and the decomposition of oxygenated graphene can be accelerated by shear, where the reaction rate increases exponentially with shear stress (
1). This idea suggests that it should be possible to use an atomic force microscope (AFM) tip to form nanoscale patterns by locally inducing chemical reactions on the surface.
Such nanoscale scanning probe lithography has recently been achieved on silicon, used in the fabrication of integrated circuits, by a collaboration between the groups of professor Linmao Qian of Southwest Jiaotong University, Chengdu, China, and STLE-member professor Seong Kim of Pennsylvania State University.
The two groups demonstrated that scanning a silica microsphere AFM probe over a silicon surface in the presence of ambient air with a relative humidity of ~75% produced a localized depression that was ~1.4 Å deep (
see Figure 1a-d), close to the theoretical thickness of a single layer of silicon (
see Figure 1e).
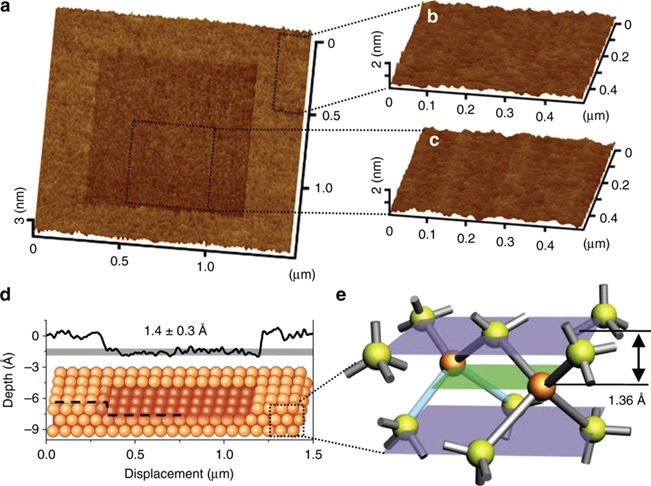 Figure 1. Single atomic layer removal of silicon. (a) AFM image (1.5 µm × 1.5 µm) of the rubbed area. Topographic images (0.5 µm × 0.5 µm) of (b) the original surface, and (c) the rubbed surface. (d) Cross-sectional profile of the rubbed area corresponding to the removal of a single atomic layer on Si(100). (e) Crystal structure of Si(100). (Published with permission from Ref. 2.)
Figure 1. Single atomic layer removal of silicon. (a) AFM image (1.5 µm × 1.5 µm) of the rubbed area. Topographic images (0.5 µm × 0.5 µm) of (b) the original surface, and (c) the rubbed surface. (d) Cross-sectional profile of the rubbed area corresponding to the removal of a single atomic layer on Si(100). (e) Crystal structure of Si(100). (Published with permission from Ref. 2.)
Control experiments were carried out in vacuum, dry air, oxygen or nitrogen atmospheres where no etching was observed. Furthermore, no silicon was removed when replacing the silicon tip by one made of diamond, leading to the conclusion that both water and the presence of a silicon counterface are required for tribochemical etching of the surface to occur.
To provide insights into the reaction mechanism, molecular dynamics (MD) simulations were carried out in collaboration with researchers at the State Key Laboratory of Tribology at Tsinghua University in China, for a silicon dioxide nanoparticle sliding against a silicon surface (
see Figure 2a), showing that the contact responds elastically when loaded in the absence of water with negligible removal of silicon from the surface (
see Figure 2b, c). The simulations also provide insights into the tribochemical reaction pathway. They reveal that the reaction is initiated by the formation of hydroxyl species by reaction with water vapor (
see Figure 2d) followed by the formation of bridges between two surface hydroxyl groups across the interface (
see Figure 2e). Sliding then induces cleavage of the substrate bonds, thereby causing a silicon atom to be removed from the surface, and elegantly explains the experimental results in which both water and a silicon counterface are required for the reaction to occur. The reaction cycle is continued by water from the atmosphere reacting with the silicon surface to reform hydroxyl groups.
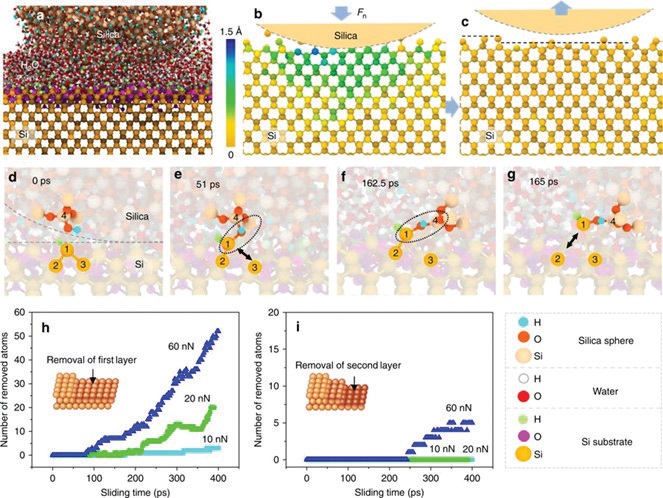 Figure 2. Molecular dynamics simulations of the atomic removal of silicon rubbing against a silica nano-sphere. (a) Sliding model of the MD simulation. (b) Deformation of the silicon substrate under load. (The z direction displacement of Si atoms under contact pressure is denoted by different colors.) (c) Removal of silicon atoms and the relaxation of the substrate after unloading. (d-g) Mechanochemical reaction process: (d) Initial contacting interface reacting with water to form Si–H and Si–OH groups (i.e., Si2–3 = Si1–H on the lower silicon substrate and O–Si4–OH on the upper SiO2 nano-sphere); (e) Formation of an Si1–O–Si4 interfacial bond bridge between the silicon substrate and the silica tip, showing cleavage of the Si1–Si3 bond; (f) Tensile stress transferred across Si1–O–Si4 bonding bridges; (g) Cleavage of the Si1–Si2 bond and atomic Si1 removal from the substrate. (h) and (i) show the number of Si atoms removed from the first atomic layer (inset schematic in h) and second atomic layer (inset schematic in i), respectively, as a function of sliding time under different load conditions. (Published with permission from Ref. 2.)
Figure 2. Molecular dynamics simulations of the atomic removal of silicon rubbing against a silica nano-sphere. (a) Sliding model of the MD simulation. (b) Deformation of the silicon substrate under load. (The z direction displacement of Si atoms under contact pressure is denoted by different colors.) (c) Removal of silicon atoms and the relaxation of the substrate after unloading. (d-g) Mechanochemical reaction process: (d) Initial contacting interface reacting with water to form Si–H and Si–OH groups (i.e., Si2–3 = Si1–H on the lower silicon substrate and O–Si4–OH on the upper SiO2 nano-sphere); (e) Formation of an Si1–O–Si4 interfacial bond bridge between the silicon substrate and the silica tip, showing cleavage of the Si1–Si3 bond; (f) Tensile stress transferred across Si1–O–Si4 bonding bridges; (g) Cleavage of the Si1–Si2 bond and atomic Si1 removal from the substrate. (h) and (i) show the number of Si atoms removed from the first atomic layer (inset schematic in h) and second atomic layer (inset schematic in i), respectively, as a function of sliding time under different load conditions. (Published with permission from Ref. 2.)
The simulations also predict that the rate of silicon removal from the first layer increases exponentially with the applied load (
see Figure 2h), and two layers of silicon are removed at even higher loads (
see Figure 2h). This exponential load dependence was confirmed experimentally and is in agreement with the load dependences for tribochemical reaction rates found previously (
1).
These elegant results illustrate how fundamental insights into the mechanisms of tribochemical reactions of lubricants and lubricant additives can have a significant influence on other fields of science.
REFERENCES
1.
Tysoe, W.T. and Spencer, N.D. (2015), “Reaction to rubbing,” TLT,
71 (8), pp. 84-86.
2.
Chen, L., Wen, J., Zhang, P., Yu, B., Chen, C., Ma. T., Lu, X., Kim, S.H. and Qian, L. (2018), “Nanomanufacturing of silicon surface with a single atomic layer precision via mechanochemical reactions,”
Nature Communications,
9, 1542.
Eddy Tysoe is a distinguished professor of physical chemistry at the University of Wisconsin-Milwaukee. You can reach him at wtt@uwm.edu.
Nic Spencer is professor of surface science and technology at the ETH Zurich, Switzerland, and editor-in-chief of STLE-affiliated Tribology Letters journal. You can reach him at nspencer@ethz.ch.