Success in the ring
Drs. Wilfred T. Tysoe & Nicholas D. Spencer | TLT Cutting Edge June 2018
A new class of sulfur- and phosphorus-free friction modifiers shows great promise in the boundary-lubrication regime.
 © Can Stock Photo / hKuprevich
© Can Stock Photo / hKuprevich
There is still, literally, a lot of mileage to be gained from improvements in lubricants for automobile engines. In particular, as lower and lower viscosities are required to save energy in the hydrodynamic-lubrication regime, the likelihood of engines spending more of their time in the boundary regime increases. This brings with it new challenges, requiring more efficient antiwear and friction-reducing additives. Complicating the picture still further in automobiles is the requirement that traces of any new additive should not poison the downstream catalytic converter.
With these daunting challenges in mind, a collaborative effort led by professors Tobin Marks, Jane Wang and Yip-Wah Chung at Northwestern University, together with colleagues at Argonne National Laboratory and Valvoline, Inc., has been working on developing a new generation of friction-reducing additives (
1), inspired by a molecule used as a friction modifier in the hard-disk industry, known as A20H (
see Figure 1).
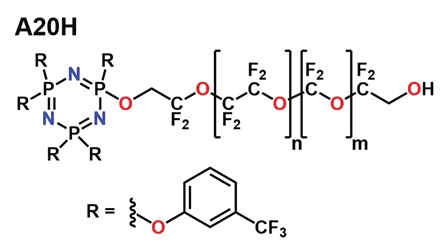 Figure 1. Molecular structure of the hard-disk-drive lubricant, A20H. (Reprinted from Ref. [1] by kind permission of Springer-Nature.)
Figure 1. Molecular structure of the hard-disk-drive lubricant, A20H. (Reprinted from Ref. [1] by kind permission of Springer-Nature.)
To lubricate well in the boundary regime, a molecule has to attach strongly to the sliding surfaces. A20H binds well to carbon in the hard-disk surface by means of multiple interactions with the ring atoms. However, for automobile applications, the phosphorus and fluorine content would immediately rule it out, due to its potential poisoning effects on the catalytic converter. Nevertheless, the idea of multiple attachment to a surface by rings can be exploited for the metal-containing surfaces of engines by applying the mechanism of
chelation, in which molecules form several bonds to a single metal ion. Thus, the goal was to discover ring-based, chelating, catalyst-compatible, friction-modifying molecules, which could adsorb from the oil onto the sliding surfaces of engines and remain there under relatively high-temperature operating conditions.
After ruling out some molecules that were unstable below 200 C, two candidate structures were chosen, triazines and cyclens (
see Figure 2), along with proposed binding interactions with the hydroxyl groups on metal surfaces.
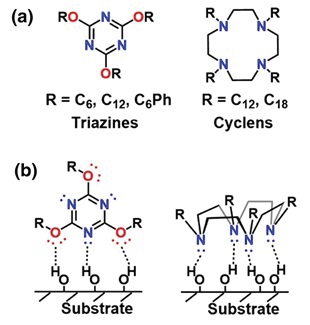 Figure 2. (a) Molecular structures of two candidate friction-modifying additives and (b) the proposed binding geometries of each additive to hydroxyl groups on metal substrates. (Adapted from Ref. [1] by kind permission of Springer-Nature.)
Figure 2. (a) Molecular structures of two candidate friction-modifying additives and (b) the proposed binding geometries of each additive to hydroxyl groups on metal substrates. (Adapted from Ref. [1] by kind permission of Springer-Nature.)
The synthesized triazines and cyclens are stable up to temperatures in excess of 300 C. Tribological tests were therefore performed with base-oil solutions of these molecules between steel surfaces under sliding and mixed sliding-rolling conditions, at pressures up to 1 GPa, and temperatures up to 200 C.
The results show that both classes of molecules show friction reduction in the boundary regime at lower temperatures. However, the triazines appear to lose their effectiveness above about 125 C. The cyclen molecules, with attached dodecyl groups (C12-cyclen), however, actually seem to become more effective at higher temperatures, reducing the friction coefficient measured from µ=0.15 (base oil) to µ=0.09 (base oil + C12-cyclen) at 75 C, and from µ=0.21 to µ=0.07 at 200 C.
Possibly the most impressive results were obtained during more than one hour of ball-on-flat, reciprocating-sliding tests of steel against steel at 1 GPa pressure and a temperature of 100 C. C18-cyclens (1 wt%) were added to a synthetic base oil (PAO4) and also to a fully formulated 5W30 engine oil (
see Figure 3). Their performance was compared to that of
Armeen T—a commercially available, alkylamine-based friction modifier—at a similar concentration. In the PAO4 case, the cyclen-containing lubricant showed lower friction compared to both the base oil and the
Armeen-T-containing oil after about 1,000 s, whereas in the fully formulated oil, the C18-cyclen-containing 5W30 oil clearly showed a significantly lower friction value than both other lubricants over the entire experiment. This shows that the cyclens are not only of theoretical interest, but their adsorption onto the sliding surfaces can even compete at 100 C with the host of additives already present in commercial oils.
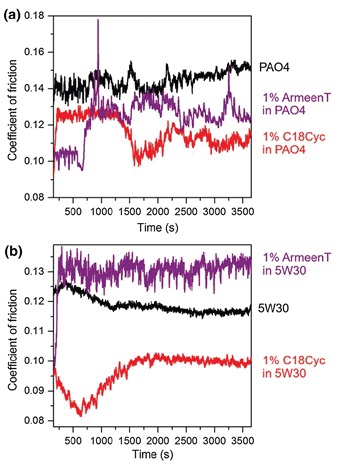 Figure 3. Ball-on-flat linear reciprocating tests at 100 C for (a) PAO4 oils with and without addition of 1 wt% C18-cyclen or 1 wt% Armeen T and (b) fully formulated 5W30 oils with and without addition of 1 wt% C18-cyclen or 1 wt% Armeen T. (Adapted from Ref. [1] by kind permission of Springer-Nature.)
Figure 3. Ball-on-flat linear reciprocating tests at 100 C for (a) PAO4 oils with and without addition of 1 wt% C18-cyclen or 1 wt% Armeen T and (b) fully formulated 5W30 oils with and without addition of 1 wt% C18-cyclen or 1 wt% Armeen T. (Adapted from Ref. [1] by kind permission of Springer-Nature.)
This piece of work is a nice example of a collaboration between, on the one hand, chemists with a thorough understanding of both synthesis and organometallic interactions, and, on the other hand, tribologists who know how to put new additives through their paces under a wide range of realistic conditions.
REFERENCE
1.
Desanker, M., He, X., Lu, J., Johnson, B.A., Liu, Z., Delferro, M., Ren, N., Lockwood, F.E., Greco, A., Erdemir, A., Marks, T.J., Wang, Q.J. and Chung, Y.-W. (2018), “High‑performance heterocyclic friction modifiers for boundary lubrication,”
Trib. Lett,
66, 50.
Eddy Tysoe is a distinguished professor of physical chemistry at the University of Wisconsin-Milwaukee. You can reach him at wtt@uwm.edu.
Nic Spencer is professor of surface science and technology at the ETH Zurich, Switzerland, and editor-in-chief of STLE-affiliated Tribology Letters journal. You can reach him at nspencer@ethz.ch.