NOx catalyst mechanism
Dr. Neil Canter, Contributing Editor | TLT Tech Beat November 2017
Oxygen-bridged copper dimers are the key to the zeolite catalyst converting NOx to nitrogen and water.
KEY CONCEPTS
• The reduction of NOx in a diesel engine is achieved through the formation of an oxygen-bridged copper dimer.
• Copper I ions within the zeolite catalyst form the complex and then move back to their original positions.
• The effectiveness of the catalyst is dependent upon how effectively copper I ions diffuse through the zeolite catalyst.
NITROGEN OXIDE (NOx) EMISSIONS ORIGINATING from a diesel engine remain a significant pollutant that needs to be minimized. A viable approach for removing NOx, known as selective catalytic reduction (SCR), was originally developed for use in thermal power plants in Japan in the late 1970s.
During the past 10 years, new SCR catalysts have been developed for use in mobile diesel engines. These catalysts require that an aqueous urea solution, stored in a tank attached to the vehicle, be added to the emissions stream. Upon hydrolysis, the ammonia formed reacts with NOx in the presence of a zeolite catalyst to produce nitrogen and water (see Figure 1).
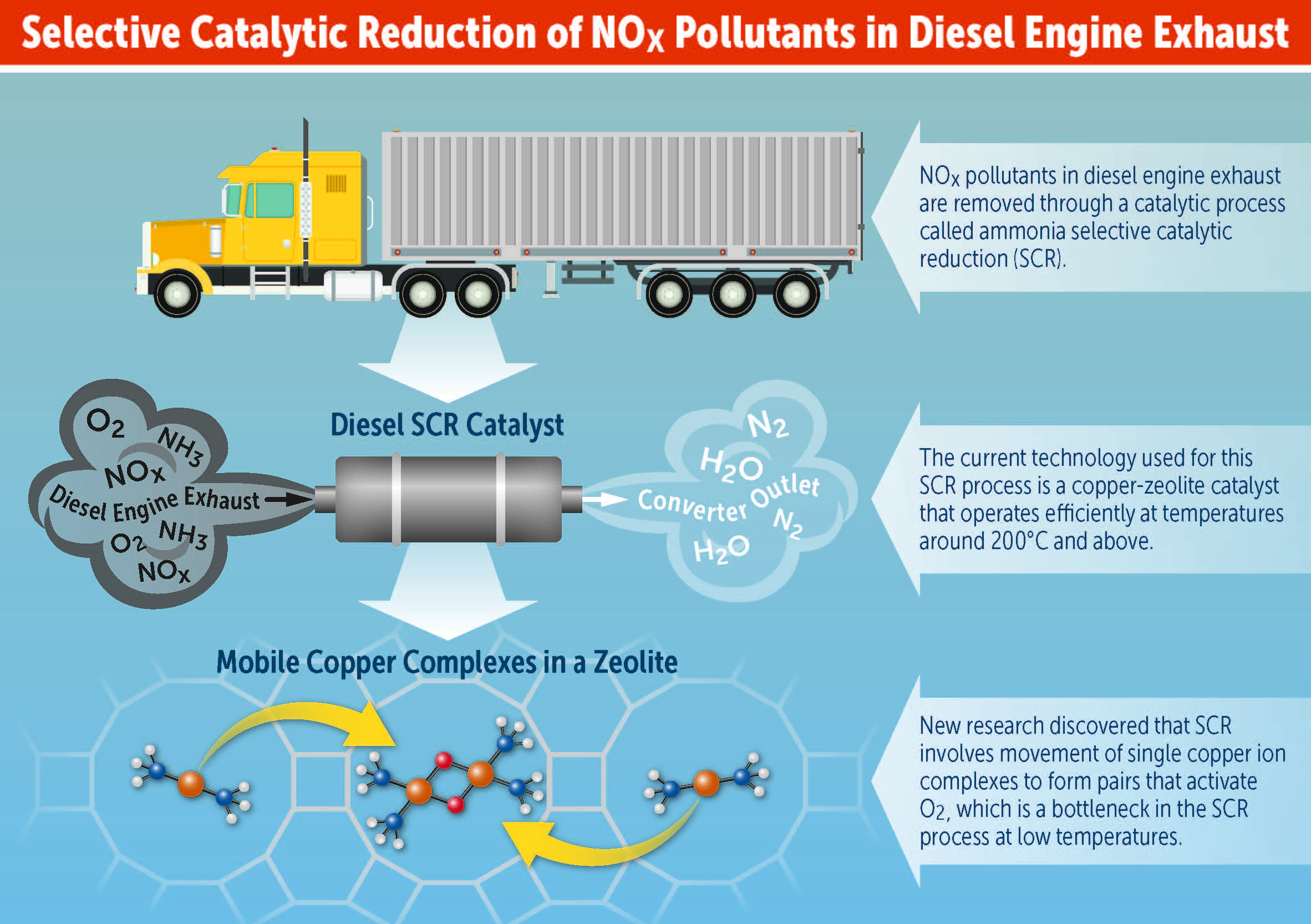
Figure 1. The formation of an oxygen-bridged copper dimer (see bottom image) is how the zeolite catalyst doped with copper II ions reduces NOx in a diesel engine. (Figure courtesy of University of Notre Dame.)
Research discussed in a past TLT article showed that SCR has been effective in reducing NOx emissions (1). In this study, a new approach was taken to measure the heavy-duty vehicle emissions by having a truck accelerate from a standing start or a crawl through a tent containing a monitoring system. The researchers found that NOx emissions coming from vehicles manufactured in 2014 were 73% lower than 2010 models.
William Schneider, H. Clifford and Evelyn A. Brosey Professor of Engineering in the department of chemical and biomolecular engineering at the University of Notre Dame in Notre Dame, Ind., says, “The zeolite catalyst used to facilitate reduction of NOx was discovered before anyone knew how it worked. The catalyst is a small-pore zeolite that is known as a chabazite. It contains atoms grouped in cages that are interconnected by six-member ring prisms and eight-member ring windows.”
Through ion exchange reactions, the zeolite catalyst is doped with copper II ions that do the actual catalysis. Schneider says, “The redox cycle that drives the reduction of NOx is the interconversion of copper I and copper II. This process is similar to homogeneous catalysis because it involves single metal ions coordinated to ammonia ligands derived from the urea. But there is uncertainty about how the actual reaction takes place. The big mystery is how does one little copper I ion consume a molecule of the oxidant, oxygen that contains four electrons per catalytic cycle?”
This question is important because the current zeolite catalyst does not perform well in the low-temperature window, below 523 K, that diesel engines often operate at. Schneider says, “Catalyst effectiveness is critical because 70%-80% of NOx emissions are generated when the engine is either warming up or idling at low temperatures.”
A greater understanding of how the copper-doped zeolite catalyst functions, particularly at low temperatures, is critical to further reducing NOx emissions. A group of researchers have now obtained further insight on the mechanism of SCR.
OXYGEN-BRIDGED COPPER DIMERS
Schneider and his students, in collaboration with researchers at Purdue and the engine manufacturer Cummins Inc., demonstrated in a recent publication (2) that copper I ions primed in specific locations within the zeolite catalyst can work in tandem to facilitate the reduction of NOx at low temperatures. He says, “We have found that the copper I ions each complexed to two ammonia molecules swim around within the catalyst as if they were loosely tethered to their home spot. When one of these mobile Cu I ions encounters a second copper I ion, the pair react with a single oxygen molecule to form an oxygen-bridged copper dimer, returning the copper I ions back to copper II.”
The bottom image in Figure 1 shows the reaction where the copper atoms are in orange, oxygen atoms are in red, the nitrogen atoms are in blue and the hydrogen atoms are in white.
Schneider says, “Once the reaction has been completed, the copper ions retreat back to where they were positioned originally. The surprising result is that the effectiveness of the catalyst does not just depend on how many active copper sites there are. It depends on how close they are and how easily they can find one another.”
The researchers used a combination of sophisticated computer models and experiments using techniques such as X-ray absorption near-edge structure spectroscopy (XANES) and extended X-ray absorption fine structure spectra (EXAFS) to demonstrate that oxygen-bridged copper dimers are the key to the zeolite catalyst converting NOx to nitrogen and water.
With the rate of the reaction directly linked to the ability of the copper I ions to diffuse through the zeolite catalyst, the researchers now are working to determine how to improve the effectiveness of the current SCR catalyst. Schneider says, “Our finding means that we now have the ability to evaluate other copper-doped zeolite catalysts to see if we can find a structural arrangement that will facilitate the movement of copper ions at low temperatures.”
A second research strategy is to focus on the oxygen-bridged copper dimer structure and determine what species present in the diesel emissions stream might interfere with its formation. Schneider says, “We will be examining which species might bind up copper sites, thereby inhibiting the formation of the oxygen-bridged dimer.”
This work establishes a framework for further research that may lead to better catalysts that can further reduce NOx emissions beyond what researchers found in truck models manufactured since 2014. Additional research can be found in a recently published paper (2) or by contacting Schneider at wschneider@nd.edu.
REFERENCES
1. Canter, N. (2015), “New method for measuring heavy-duty vehicle emissions,” TLT, 71 (4), pp. 12-13.
2. Paolucci, C., Khurana, I., Parekh, A., Li, S., Shih, A., Li, H., Iorio, J., Albarracin-Caballero, J., Yezerets, A., Miller, J., Delgass, W., Ribeiro, F., Schneider, W. and Gounder, R. (2017), “Dynamic multinuclear sites formed by mobilized copper ions in NOx selective catalytic reduction,” Science, 357 (6354), pp. 898-903.

Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be submitted to him at neilcanter@comcast.net.