Enhanced capacitor performance using natural precursor
Dr. Neil Canter, Contributing Editor | TLT Tech Beat October 2017
A carbon material with a very high electrochemically active surface area and pore volume was prepared from milk.
KEY CONCEPTS
•
Use of supercapacitors as a power source are limited due to their inability to store high levels of energy.
•
Preparation of a pure carbon source with a high electrochemically active surface area that can operate with an aqueous electrolyte has been accomplished from a natural source, non-fat powdered milk.
•
The important step in the process is a partial carbonization that preserves the nanocluster arrangement between calcium phosphate nanoclusters and casein proteins that are present in milk.
THE QUEST FOR ALTERNATIVE POWER SOURCES has focused mainly on figuring out how to improve the performance, safety and durability of batteries. One other source of power that can be used in a similar manner to the battery is the supercapacitor that stores energy with an electrochemical double layer positioned between the electrode and the electrolyte.
One problem with capacitors is their inability to store high levels of energy. In a previous TLT article, researchers developed a new type of dielectric capacitor based on nanoporous aluminum oxide (
1). The electrodes used are carbon nanotubes grown within the nanoporous aluminum oxide. A three-dimensional interdigital electrode structure was produced that lead to higher energy storage.
The current supercapacitors still utilize a salt dissolved in an organic electrolyte. David Mitlin, professor and GE chair in oil and gas systems at Clarkson University in Potsdam, N.Y., says, “A more environmentally friendly approach using an aqueous electrolyte is desirable to improve safety and reduce costs.”
One other consideration is identifying a suitable carbon-based electrode that will work in an aqueous environment to reduce costs and increase the cyclability of the supercapacitor. Currently metal oxides and nitrides such as manganese dioxide and vanadium nitride are used. Mitlin says, “Commercial supercapacitors operate with heteroatom-rich carbon sources that contain elements such as nitrogen, oxygen and phosphorus tend to degrade under high cycling rates leading to the loss of storage capacity.”
A need exists to develop a pure carbon source that exhibits a high electrochemically active surface area and can operate with an aqueous electrolyte. Such a source has now been developed from a well-known natural product, milk.
PARTIAL CARBONIZATION
Mitlin and his co-workers developed a process to produce a carbon material with a very high electrochemically active surface area and pore volume from milk. He says, “We found in milk a readily available natural source of carbon that is in plentiful supply because millions of liters have been discarded as waste in the U.S. over the past few years.”
The pyrolysis process used to convert milk to a highly porous carbon is conducted through an intermediate step. Mitlin says, “We took non-fat powdered milk and partially pyrolyzed it at a temperature of 450 C for two hours in a horizontal tube furnace under inert flowing argon. Once the partially carbonized material is isolated, it is mixed with the activating agent, potassium hydroxide and then further pyrolyzed for two more hours at temperatures ranging from 750-950 C.”
The resulting fully pyrolyzed carbon exhibits an electrochemically active surface area of 4,000 square meters per gram. This material has been designated as Immense Surface Area Carbons (ISACs) and has particle sizes in the 25-micrometer range.
In contrast, carbon obtained through conventional pyrolysis techniques displays lower surface areas. Mitlin says, “The surface area of a typical carbon used in a supercapacitor is 25% lower or 1,000 square meters per gram. We also evaluated an advanced electrode-grade activated carbon with a surface area of 2,000 square meters per gram.”
ICP and X-ray photoelectron spectroscopy analysis shows that ISACs contain lower levels of heteroatoms than are found in carbon obtained through pyrolyzing carbon by other techniques.
Milk works effectively as the carbon source because of the presence of casein protein micelles. This natural material’s characteristic bluish-white macroemulsion consists of casein protein micelles dispersed in the continuous liquid phase. The casein protein micelles contain four types of proteins that interact with calcium phosphate nanoclusters to self-assemble into highly stable micellular structures (
see Figure 2).
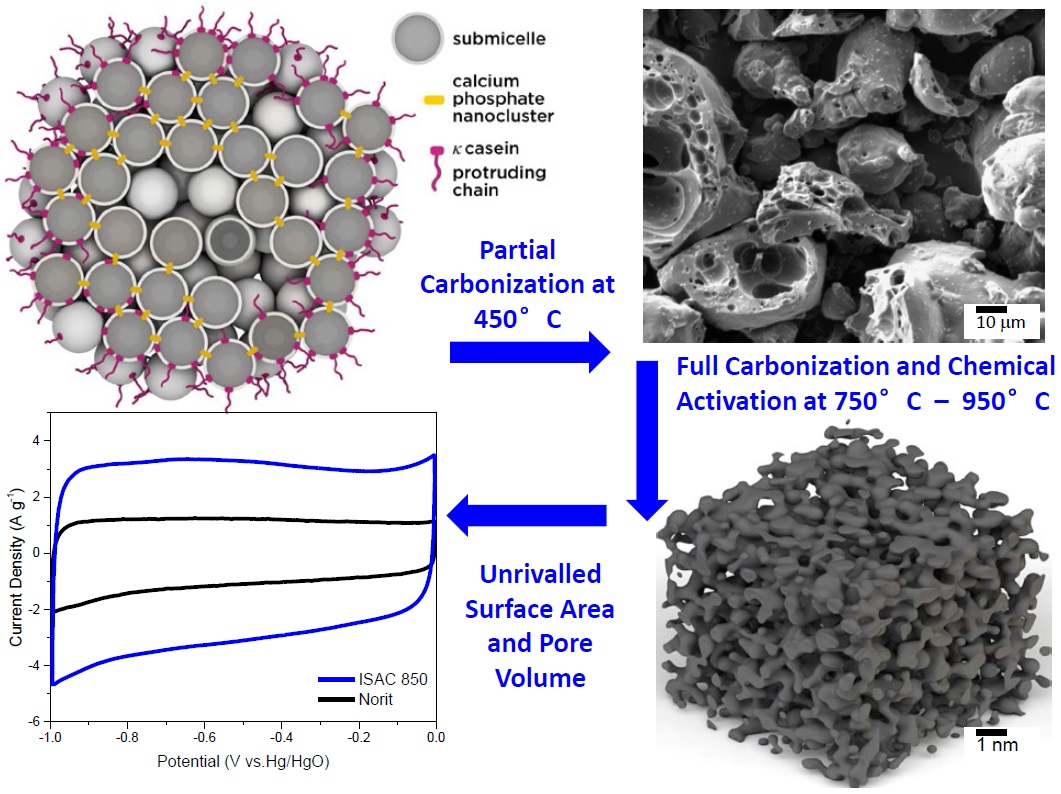 Figure 2. The process for converting the casein and calcium phosphate nanoclusters present in milk (see top left image) to ISACs (see bottom right image) through partial carbonization and then full carbonization steps is shown. Electrochemical testing shows that the ISACs exhibit superior capacitance as compared to currently used carbon materials. (Figure courtesy of Clarkson University.)
Figure 2. The process for converting the casein and calcium phosphate nanoclusters present in milk (see top left image) to ISACs (see bottom right image) through partial carbonization and then full carbonization steps is shown. Electrochemical testing shows that the ISACs exhibit superior capacitance as compared to currently used carbon materials. (Figure courtesy of Clarkson University.)
The key to the process is the researchers’ hypothesis that the partial carbonization process preserves the nanocluster arrangement seen in milk between calcium phosphate nanoclusters and casein proteins as shown in Figure 2. This forms the basis for the three-dimensional nano-scale structure that is produced after complete carbonization.
Mitlin says, “When milk is completely pyrolyzed using a conventional process, the resulting carbon material does not exhibit the high surface area obtained through our partial carbonization process.”
ISACs were evaluated in supercapacitors by using a series of electroanalytical techniques. Three-electrode and two-electrodes systems were used with two aqueous electrolytes, potassium hydroxide and lithium sulfate. Mitlin says, “We found that ISACs display 1.5 times higher capacitance as compared to currently used carbon materials. The durability of the supercapacitors containing ISACs reached 100,000 cycles with only minimum degradation. In comparison, heteroatom-rich carbon sources will only operate effectively for 10,000 cycles.”
Figure 2 shows an electrochemical profile of ISAC as compared to a high surface area activated carbon known as Norit.
Future work will involve scaling up the process for pyrolyzing ISACs so that they can be used in supercapacitors. Mitlin says, “We believe it is readily feasible to scale up ISAC production from the gram to the kilogram scale.”
The researchers also are looking at other applications for ISACs. Mitlin says, “With their high surface area, ISACs may be useful as catalysts and also for use in thermal insulation applications.” Additional information can be found in a recent article (
2) or by contacting Mitlin at
dmitlin@clarkson.edu.
REFERENCES
1.
Canter, N. (2016), “Improving capacitor storage capacity,” TLT,
72 (1), pp. 14-15.
2.
Pokrzywinski, J., Keum, J., Ruther, R., Self, E., Chi, M., Meyer, H., Littrell, K., Aulakh, D., Marble, S., Ding. J., Wriedt, M., Nanda, J. and Mitlin, D. (2017), “Unrivaled combination of surface area and pore volume in micelle-templated carbon for supercapacitor energy storage,”
Journal of Materials Chemistry A,
5, pp. 13511-13525.
 Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net.