Non-flammable graphene oxide
Dr. Neil Canter, Contributing Editor | TLT Tech Beat July 2017
Treatment with an aluminum salt may lead to further applications.
KEY CONCEPTS
•
Graphene oxide is a widely used derivative of graphene because it can easily be chemically modified but can exhibit exceptional flammability.
•
Cross-linking graphene oxide with an inorganic aluminum salt significantly reduces concern about flammability.
•
Aluminum cations reduce flammability by reacting with highly flammable graphene oxide derivatives and by shielding heat propagation between the graphene oxide flakes.
RESEARCH ON GRAPHENE, two-dimensional sheets of carbon organized into hexagonal honeycombs, and its derivatives has been widely covered in this column because this material exhibits excellent lubricity and antiwear characteristics. Much of this effort has involved evaluating graphene as a solid lubricant.
In a previous TLT article, a method developed to disperse graphene into a liquid medium was discussed (
1). This process was accomplished by dispersing graphene oxide in water followed by capillary compression and heat leading to the formation of crumpled graphene balls. Dispersion into 4 cSt polyalphaolefin (PAO) occurred through sonication. Lower friction and wear results were found with graphene dispersed at low treat rates in PAO compared to a commercially available 5W-30 engine oil.
Graphene oxide is widely used as a key graphene derivative because it can easily be chemically modified with oxygenated functionalities such as hydroxyl and carboxylic acid groups. But graphene oxide is exceptionally flammable, potentially limiting its future use in commercial applications.
Graphene oxide is prepared directly from graphite through a technique known as the Hummers method. Graphite is oxidized with a combination of potassium permanganate and potassium persulfate followed by exfoliation of the negatively charged graphene oxide.
Ryan Tian, associate professor of inorganic chemistry at the University of Arkansas in Fayetteville, Ark., says, “During the preparation of graphene oxide, byproducts from the Hummers method turned graphene oxide into a highly flammable material.”
Tian indicates that buildup of too much negative charge on the surface of the graphene oxide also is detrimental. He adds, “Upon exposure of the graphene oxide to a flame, the material burnt very quickly due to rapid flame propagation.”
Attempts to eliminate the flammability of graphene oxide through physical techniques have proven to be difficult. Filtration and washing with pure water was ineffective. The first technique did not work because graphene oxide flakes easily plug up filter pores. Water washing proved to be troublesome because repeated use caused graphene oxide to gel increasing the difficulty of further processing through centrifugal separation.
The ineffectiveness of physical techniques means that another approach is needed to minimize flammability of graphene oxide. A chemical modification technique has now been developed and proven as low cost and effective.
CROSS-LINKING
Tian and his colleagues found that cross-linking graphene oxide with an inorganic aluminum salt produces a material that is not flammable. He says, “We selected an aluminum cation because this species has inherent flame retardant properties and strongly coordinates with oxygen atoms that are present on the surface of graphene oxide.”
One of the oxygenated species found to be a major source of flammability in graphene oxide is epoxides. Aluminum cations can react to open the epoxide ring making them less flammable.
The researchers reacted a suspension of exfoliated graphene oxide in water with aluminum nitrate at room temperature. The cross-linking occurred instantly and after washing with water, the resulting cross-linked graphene oxide was drop-cast on a glass-slide membrane to form a transparent membrane.
A commercial lighter burning butane fuel generated an open flame that attempted to burn the 15- to 20-micron thick membrane of cross-linked graphene oxide. But no combustion was seen.
Figure 2 shows a comparison of graphene oxide and cross-linked graphene oxide after exposure to a flame for eight seconds. Clearly there is a major difference as the graphene oxide is readily flammable and the cross-linked graphene oxide is not.
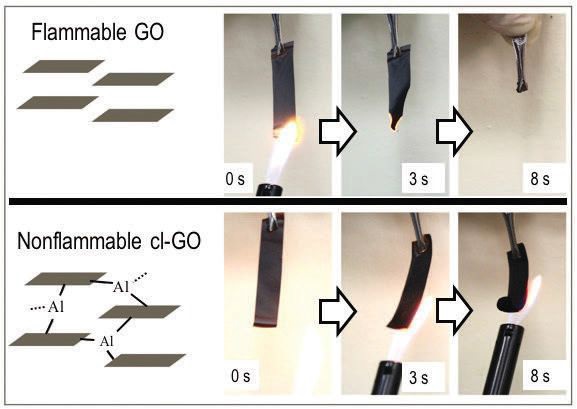 Figure 2. The flammability of graphene oxide is significantly retarded through cross-linking with aluminum cations as shown when both materials are exposed to an open flame. (Figure courtesy of the University of Arkansas.)
Figure 2. The flammability of graphene oxide is significantly retarded through cross-linking with aluminum cations as shown when both materials are exposed to an open flame. (Figure courtesy of the University of Arkansas.)
Thermogravimetric analysis and differential scanning calorimetry were conducted to evaluate the thermal decomposition process for both graphene oxide and cross-linked graphene oxide. With graphene oxide, thermal decomposition is much more exothermic due to deoxygenation triggering combustion. In contrast, cross-linked graphene oxide reduces the epoxide content limiting the exotherm.
Tian says, “The change in flammability is achieved with a small percentage of aluminum by weight. As soon as we add the aluminum cation, the flammability is diminished.”
Besides reacting with epoxides, the researchers also believe that the aluminum cations minimize flammability by shielding heat propagation between the graphene oxide flakes.
FTIR and powder X-ray diffraction data confirm that the aluminum cations react with epoxides on the surface of the graphene oxide. Tian believes that chemical modification of graphene oxide can be done with other metals to produce materials that will display no flammability and can be used in a wide range of commercial applications including solar cells, supercapacitors and sensors.
Tian says, “We are evaluating the use of transition metal and rare-earth metal cations with three or more positive charges. The d and f orbitals in these metals open up new opportunities besides minimizing flammability.”
Tian also feels that this strategy can be used to cross-link other two-dimensional materials such as hexagonal boron nitride and molybdenum disulfide. He says, “This low-cost technique may provide the opportunity to tailor advanced materials for high-performance applications.
Additional information can be found in a recent article (
2) or by contacting Tian at
rtian@uark.edu.
REFERENCES
1.
Canter, N. (2016), “Evaluation of a new lubricant additive: Crumpled graphene balls,” TLT,
72 (4), pp. 12-13.
2.
Turgut, H., Tian. Z., Yu, F. and Zhou, W. (2017), “Multivalent cation cross-linking suppresses highly energetic graphene oxide’s flammability,”
The Journal of Physical Chemistry C,
121 (10), pp. 5829-5835.
 Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net.