Nano-based molten inorganic salts
Dr. Neil Canter, Contributing Editor | TLT Tech Beat May 2017
Nanoparticles derived from metals and semiconductors can form stable dispersions in molten inorganic salts for the first time.
KEY CONCEPTS
•
Metal and semiconductor quantum dot nanocrystals were stabilized in molten inorganic salts for the first time.
•
The researchers believe the nanocrystalline dispersions are stabilized through a partial ordering of the molten inorganic salt.
•
The nucleophilicity of an anion used in the molten salt may play a role in determining whether a stable dispersion can be formed with the nanocrystals.
A CHALLENGE IN USING NANOADDITIVES in base fluids is finding a way to stabilize them so they do not precipitate out of the dispersion initially formed. Nanoadditives are under evaluation in a number of lubricant applications.
One use discussed in a past column is in transformer oils (
1). This fluid type must keep the transformer cool and provide electric insulation between internal live parts. Researchers added hexagonal boron nitride at a concentration of 0.1% in a dispersion in naphthenic base oil and realized a thermal conductivity enhancement of 80%. This improvement is due to the two-dimensional hexagonal boron nitride providing a percolation channel facilitating movement of electrons and also exhibiting Brownian motion to facilitate heat removal.
Another application where nanoadditives have enhanced performance is in heat-transfer fluids. One type of heat transfer fluid that is used in applications such as solar energy is molten inorganic salts. By definition, molten inorganic salts are solids at room temperature but will melt at higher temperatures and can store energy until needed in a molten state. One conventional molten salt combination of sodium nitrate and potassium nitrate can operate at temperatures up to 550 C.
But no effort has been made to determine if additizing a molten inorganic salt will lead to a performance benefit similar to what is observed with liquid base stocks. Dmitri Talapin, professor of chemistry at University of Chicago in Chicago, developed interest in trying to additize molten salts from work done in two areas. He says, “Over the past 10 years, we have been developing inorganic surfactants to prepare colloidally stable nanomaterials for applications such as quantum dots used in television displays.”
Talapin continues, “A second area involved determining how to grow quantum dots of gallium arsenide crystals by solution chemistry. Gallium arsenide is the second most important semiconductor used in solar cells, but we could not prepare it in the form of soluble quantum dots because no traditional solvents could sustain 600-700 C temperatures. Such temperatures are required to get rid of structural defects in the gallium arsenide lattice.”
Talapin and his colleagues found that nanoparticles derived from metals and semiconductors can form stable dispersions in molten inorganic salts for the first time. In addition, they found that molten salts can be used as solvents to work with gallium arsenide quantum dots.
PARTIAL ORDERING OF MOLTEN INORGANIC SALT
The researchers stabilized various nanoparticles in molten inorganic salts through the use of several techniques at elevated temperatures. One approach interfaced colloidal nanocrystals dispersed in an organic solvent such as n-decane with a molten salt such as the combination of aluminum trichloride, sodium chloride and potassium chloride. The organic solvent vaporized at the high temperature, enabling the nanocrystals to form a stable dispersion in the molten inorganic salt. Direct interaction with the nanocrystal and the molten inorganic salt also produced the desired stable dispersion at elevated temperatures. All procedures were conducted in a nitrogen-filled glove box to minimize potential contamination.
Nanocrystals derived from metals such as platinum, semiconductor quantum dots such as indium phosphide (INP) and cadmium selenide/cadmium zinc sulfide were used in combinations with six molten inorganic salts. Talapin says, “The size of the nanocrystals ranged from three to 20 nanometers in diameter.”
Nanocrystals were present at concentrations between five and 50 milligrams per milliliter in the stable dispersions. Several analytical techniques were used to determine the structure of the nanocrystalline dispersions. While X-ray scattering and X-ray diffraction analysis showed no difference in the size and shape of the nanocrystals, FT-IR revealed significant differences.
Talapin says, “The two traditional mechanisms proposed for stabilizing dispersions such as liquid-liquid emulsions are steric and electrostatic. Steric factors are evident when two particles try to come close but are repelled based on factors such as osmotic pressure. Electrostatic issues focus on whether the nanoparticle surfaces exhibit electric charge. Repulsion is present if nanoparticles with the same charge approach too closely enable them to remain in a stable dispersion.”
Talapin believes that a third mechanism is present, which allow for partial ordering of the molten inorganic salt. He says, “This partial ordering is dependent upon the type of molten salt used. For example, platinum nanocrystals show a chemical affinity to the chloride anion present in the blend of aluminum trichloride, sodium chloride and potassium chloride leading to the surface of the nanocrystals being decorated with chloride anions in a stable dispersion as shown on the right side of Figure 2. Beyond the chloride anions is a layer of cations attracted electrostatically to them. This quasi-ordered arrangement extends into the molten inorganic salt phase for two nanometers beyond the nanocrystal leading to a stable dispersion where the nanocrystals cannot agglomerate.”
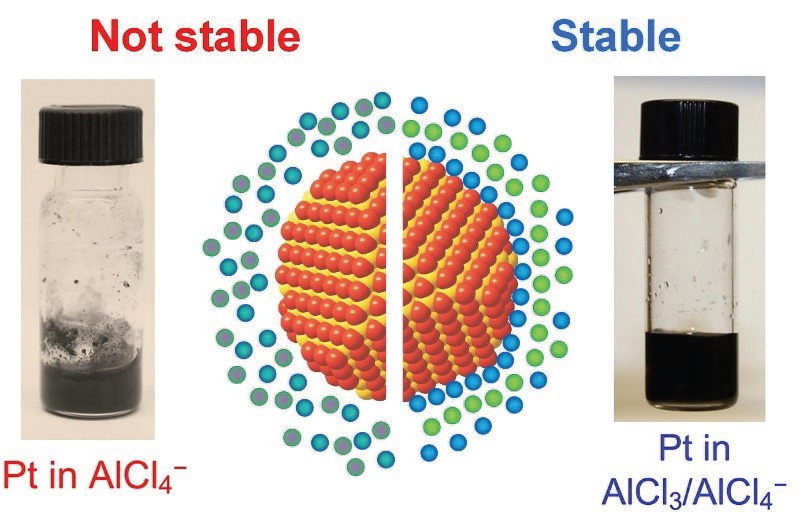 Figure 2. Chemical affinity of the nanoparticle surface (represented by orange spheres) to species in molten salt (represented by blue and green spheres) are necessary for colloidal stability. Corresponding examples of stable and unstable colloidal dispersions of platinum nanocrystals are shown in the photographs. Stable solution: Pt nanocrystals in AlCl3/NaCl/KCl molten salt containing excess of AlCl3 that bind to the nanoparticle surface. Unstable solution: same nanocrystals in AlCl3/NaCl/KCl molten salt where the composition was adjusted to contain only chemically inert Na+, K+, and AlCl4- ions. (Figure courtesy of University of Chicago.)
Figure 2. Chemical affinity of the nanoparticle surface (represented by orange spheres) to species in molten salt (represented by blue and green spheres) are necessary for colloidal stability. Corresponding examples of stable and unstable colloidal dispersions of platinum nanocrystals are shown in the photographs. Stable solution: Pt nanocrystals in AlCl3/NaCl/KCl molten salt containing excess of AlCl3 that bind to the nanoparticle surface. Unstable solution: same nanocrystals in AlCl3/NaCl/KCl molten salt where the composition was adjusted to contain only chemically inert Na+, K+, and AlCl4- ions. (Figure courtesy of University of Chicago.)
Adjusting the molten salt so that the main anion is tetrachloroaluminate leads to an unstable dispersion as shown on the left side of Figure 2. Talapin explains, “Tetrachloroaluminate is not as nucleophilic as chloride, which means that this anion does not bind to the surface of the platinum nanocrystal.” The same observation was made for nitrate salts that also do not form stable dispersions.
Talapin indicates that some of the nanocrystalline dispersions in molten inorganic salts are stable for weeks while others are stable for a shorter period. He says, “Differences may be due to the lower thermal stability of some nanocrystals.”
Future work will focus on determining the chemical structures forming at surfaces of nanocrystals that can form stable dispersions. For applications such as heat transfer fluids, a higher concentration means greater heat transfer. The microscopic origin of the colloidal stability also will be investigated.
Additional information can be found in a recent paper (
2) or by contacting Talapin at
dvtalapin@uchicago.edu.
REFERENCES
1.
Canter, N. (2012), “Nano-based transformer insulating fluid,” TLT,
68 (5), pp. 10-11.
2.
Zhang, H., Dasbiswas, K., Ludwig, N., Han, G., Lee, B., Vaikuntanathan, S. and Talapin, D. (2017), “Stable colloids in molten inorganic salts,”
Nature,
542 (7641), pp. 328-331.
 Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net.