Presence of carbon nanotubes in automobile emissions
Dr. Neil Canter, Contributing Editor | TLT Tech Beat February 2017
An initial study shows that sulfur and iron need to be in the fuel at specific levels to lead to the formation of CNTs.
KEY CONCEPTS
•
The presence of sulfur in fuel and iron in fuel additives led to the hypothesis that single-walled carbon nanotubes (CNTs) might be formed during combustion in a diesel engine.
•
Initial studies show that sulfur levels above what is present in diesel fuels used in the EU and North America lead to the formation of CNTs.
•
Nothing present in the diesel engine oil contributes to the formation of CNTs.
REDUCING THE EMISSIONS ORIGINATING FROM THE INTERNAL COMBUSTION ENGINE of an automobile remains a high priority for the automotive industry. Much progress has been made during the past five years with emissions from heavy-duty vehicles built since 2011 declining by more than a factor of 30 compared to the same type of vehicle built before 2007 due to the presence of diesel particulate filters (
1).
But there is concern that improvements in automobile technologies that are needed to improve fuel economy might lead to an increase in emissions. In a previous TLT article, research indicated that an increase in the use of gasoline direct injection engines used to improve fuel economy might lead to an increase in black carbon emissions (
2).
Single-walled carbon nanotubes (CNTs) were discovered about 25 years ago and are 100 times stronger than steel (per unit mass). They exhibit superior mechanical and electrical properties.
Jacob Swanson, assistant professor in the department of integrated engineering at Minnesota State University in Mankato, Minn., says, “CNTs are cylindrical structures of carbon with diameters ranging from one to 20 nanometers and lengths between 50 and 10,000 nanometers, giving them very high aspect ratios (ratio of length versus width).”
The biggest concerns about CNTs are related to their similarity to asbestos in structure. Swanson says, “CNTs have high aspect ratios and fibrous morphology, which makes them similar to mineral-like fibers that if inhaled have been the cause of lung disease. Mice and rat studies have shown the detrimental effects of CNTs.”
But Swanson points out that no single health effect related to people has been reported through exposure to CNTs.
Swanson decided to examine the possible formation of CNTs in automotive emissions based on two issues that converged. He says, “Anecdotal evidence and information present in the literature shows there is a possibility for CNTs to form in internal combustion engines. We gained insight on producing CNTs from past work done to evaluate their synthesis from the reaction of hydrocarbons, a sulfur source and iron in a tube furnace reactor operated at high temperatures. This gas phase reaction is similar to what is occurring in an internal combustion engine.”
Based on previous work, Swanson hypothesizes that the sulfur content of fuel and the presence of iron will impact the formation of CNTs in an internal combustion engine. Research has now been accomplished that identifies CNT formation from studies done on a diesel engine.
HIGH SULFUR LEVEL
In experiments with a four-stroke, single-cylinder, overhead valve diesel engine that is air-cooled, Swanson and his colleagues found evidence for CNTs under specific operating conditions where the level of sulfur present in the fuel is 4,500 ppm and iron is present at a concentration of 36 ppm. To simulate the conditions found in the gas phase reactions, the researchers used ferrocene as the iron source because this organometallic compound has been used as a catalyst additive in diesel fuels. A dynamometer was connected to the engine in order to better control speed and load and the engine was operated at two speeds (1,800 and 2,400 rpm).
Swanson says, “There is a historical precedent for the use of iron additives in diesel fuel to reduce soot formation.”
The researchers evaluated four other operating levels of sulfur and iron with zero levels of each element acting as the control. The source of the sulfur was a combination of benzothiophene and dibenzothiophene that are two of the most common forms of sulfur found in diesel fuel. These compounds are added to standard ultra-low sulfur diesel fuel that contains no more than 10 ppm of sulfur.
In testing at lower sulfur levels (0 and 500 ppm) and either in the presence or absence of iron, no evidence of CNTs was seen from transmission electron microscopy (TEM) analysis of emission samples taken during the engine tests.
Swanson says, “We felt that the best initial approach was to run the engines under moderate load and speed conditions. However, additional research is needed to understand the effects of these variables on CNT formation because they effect combustion properties such as time of combustion. Other engines operate at faster or slower speeds. For example, marine engines operate at a factor of 10 lower speed than automobile engines leading to a much different time-temperature history. This may or may not lead to prevailing conditions that are more conducive to CNT formation.”
Swanson says, “The CNTs identified are shorter and less tangled with estimated diameters of 15 nanometers and lengths of 100 nanometers.”
Figure 1 shows a TEM image of a carbonaceous, diesel aggregate near two CNT particles that are perpendicular to each other. Swanson hypothesizes that CNTs develop from iron catalyst particles.
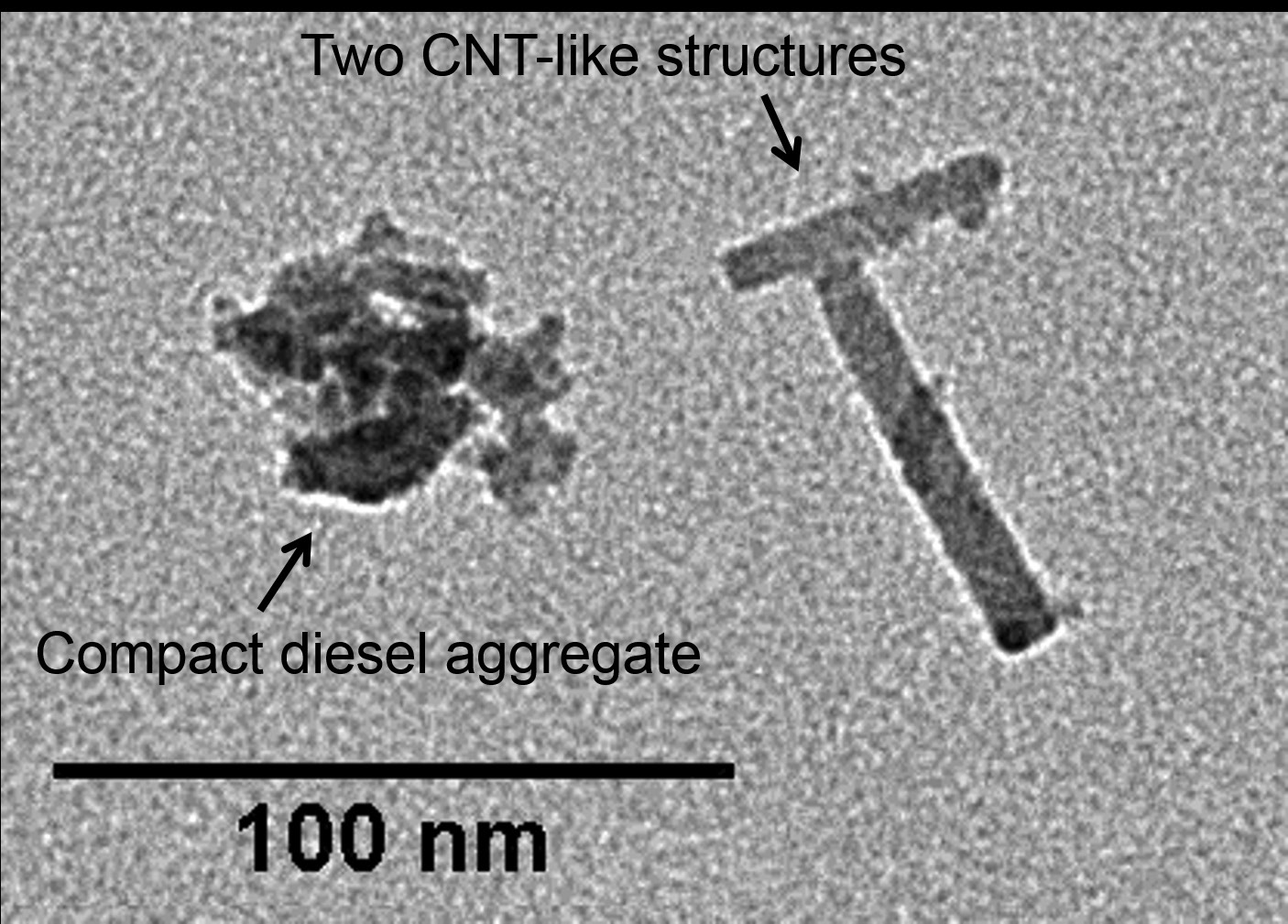 Figure 1. A compact, carbonaceous, diesel aggregate located nearby two crossed CNT-like structures on a transmission electron microscopy grid is shown. The dark contrast area on the end of one CNT-like structure is indicative of an iron seed particle that initiated the CNT growth process. (Figure courtesy of Minnesota State University.)
Figure 1. A compact, carbonaceous, diesel aggregate located nearby two crossed CNT-like structures on a transmission electron microscopy grid is shown. The dark contrast area on the end of one CNT-like structure is indicative of an iron seed particle that initiated the CNT growth process. (Figure courtesy of Minnesota State University.)
In running these experiments, the researchers used CJ-4 approved diesel engine lubricants. Swanson does not believe that the lubricant contributes to the formation of CNTs. He says, “The engine used in our experiments had a reasonably high oil consumption and, thus, we implicitly evaluated how metals such as calcium and zinc that are present in engine additive packages influenced the formation of CNTs. Our data suggests that neither metal acts as a catalyst to facilitate the production of CNTs.”
The level of sulfur where CNTs are produced is not present in diesel fuels used in the EU and North America. Swanson points out that high sulfur levels are seen in some countries in the developing world. Furthermore, the diesel particulate filters currently used with modern diesel engines are extremely effective in filtering CNTs.
Future work will involve determining if other parameters such as lower engine speeds and different fuel and engine lubricants also can influence the formation of CNTs. Additional information can be found in a recent article (
3) or by contacting Swanson at
jacob.swanson@mnsu.edu.
REFERENCES
1.
Canter, N. (2015), “New method for measuring heavy-duty vehicle emission,” TLT,
71 (4), pp. 12-13.
2.
Canter, N. (2016), “Potential trade-off between fuel economy and particulate emissions,” TLT,
72 (10), pp. 16-18.
3.
Swanson, J., Febo, R., Boies, A. and Kittelson, D. (2016), “Fuel sulfur and iron additives contribute to the formation of carbon nanotube-like structures in an internal combustion engine,”
Environmental Science & Technology Letters,
3 (10), pp. 364-368.
 Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net.