Biofuel production using syngas fermentation
Dr. Neil Canter, Contributing Editor | TLT Tech Beat January 2017
An acetogenic bacterium can convert a mixture of carbon monoxide, hydrogen and carbon dioxide into organic molecules such as ethanol.
KEY CONCEPTS
•
Specific anaerobic bacteria can produce ethanol in a biological process known as syngas fermentation from carbon monoxide, hydrogen and carbon dioxide.
•
The challenge in maximizing ethanol production is that it’s not the favored process within the bacterium cell.
•
A thermodynamic shift occurs when certain nutrients are not present that forces the bacterium to shift to ethanol production.
ETHANOL HAS BECOME AN IMPORTANT COMPONENT in gasoline. According to the Department of Energy, nearly 97% of all gasoline manufactured in the U.S. contains ethanol. The typical blend known as E10 contains 10% ethanol.
High demand for biofuels combined with the desire to use renewable resources has encouraged research to find ways for maximizing ethanol production. In a past TLT article, researchers examined how to optimize the use of zeolite catalysts that are commonly used in petroleum oil processing and ethanol manufacture (
1). The approach taken was to screen over 300,000 zeolites through a multi-step computational screening to find which ones will facilitate the separation of water from ethanol, which can be a troublesome purification step. Of the 12 catalysts identified as promising, eleven were using this process.
Biotechnology also is being used to develop more efficient processes for ethanol production. Ludmilla Aristilde, assistant professor of biological and environmental engineering at Cornell University in Ithaca, N.Y., says, “One biological approach that is showing promise in manufacturing ethanol is syngas fermentation. Certain anaerobic bacteria can ferment this gas mixture containing carbon monoxide, hydrogen and carbon dioxide into organic molecules such as acetic acid and ethanol through a series of enzymatic reactions in their metabolism.”
This biotechnology process is analogous to the Fischer-Tropsch that converts carbon monoxide and hydrogen (synthesis gas) to hydrocarbons including highly refined base oils.
The type of bacteria used in syngas fermentation is known as acetogenic bacteria. Aristilde says, “Acetogenic bacteria have the metabolic capability of producing acetates by using carbon dioxide as the raw material.”
In general, bacteria of this type generate energy from two metabolic states known as acidogenesis and solventogenesis. During acidogenesis, cells generate acids such as acetate and n-butyrate from sugars. During solventogenesis, cells converting sugars into solvents including ethanol, n-butanol and acetone.
A popular type of bacterium used in these processes is
Clostridium ljungdahlii. Aristilde says, “Researchers have found that this bacterium is effective at turning inorganic species such as carbon monoxide and hydrogen into organic compounds that are commercially attractive through the use of microbial biotechnology.”
One of the challenges faced in using
Clostridium ljungdahlii to make ethanol is that solventogenesis is not a favored process within the bacterium cell. During acidogenesis, the cell will produce three moles of the energy source, ATP per one mole of the sugar, glucose while solventogenesis yields just two moles of ATP.
Aristilde says, “Acidogenesis and solventogenesis can be considered both competitive and complementary metabolic pathways. The bacterium will utilize acidogenesis as the favored pathway until no more raw material is available. Then it moves into a starvation state where it is stressed and needs to respond to generate energy by doing solventogenesis.”
Aristilde and her colleague Lars Angenent, professor of biological and environmental engineering at Cornell University, have now gained a better understanding of how
Clostridium ljungdahlii move from acidogenesis to solventogenesis.
THERMODYNAMICS
The Angenent Lab separated acidogenesis and solventogenesis by running each process in a separate bioreactor using
Clostridium ljungdahlii. Acetate and ethanol were each produced under steady-state conditions. The gaseous stream employed in both cases contained 60% carbon monoxide, 35% hydrogen and 5% carbon dioxide.
Samples were then taken and evaluated by doing a protematic mapping to analyze the enzymes found in the acidogenesis and solventogenesis samples. The Angenent Lab identified 1,988 enzymes in both samples and were surprised to find no difference in the levels seen. Aristilde says, “This was unusual because changes in enzyme levels usually are found in different growth conditions.”
The conclusion found from the protematic mapping prompted the researchers to evaluate the metabolic state of the bacterium undergoing each process to determine if nutritional components in both bioreactors influence the metabolic activity of
Clostridium ljungdahlii. The Aristilde Lab performed a metabolome analysis to determine what factors may enable the bacterium to metabolically shift from acidogenesis to solventogenesis. Aristilde says, “The sample provides a chemical snapshot of what is in the bacterium cell.”
The researchers found that limitations in certain nutrients forced the bacterium to direct reducing equivalents and carbon away from biomass production and toward the manufacture of ethanol through solventogenesis. Aristilde says, “In much the same manner that a certain concentration of a specific reactant will force a thermodynamic shift in a reaction from one direction to another, when sufficient reducing equivalents are present, the cell will deal with this stress by producing ethanol.”
The researchers developed an overflow model to explain this thermodynamic shift toward ethanol. Figure 3 shows the model, which displays the two metabolic pathways that the bacterium uses to generate energy. The biomass pathway is favored until the cell can no longer grow and biomass cannot be produced. Then the ethanol-generating pathway is preferred.
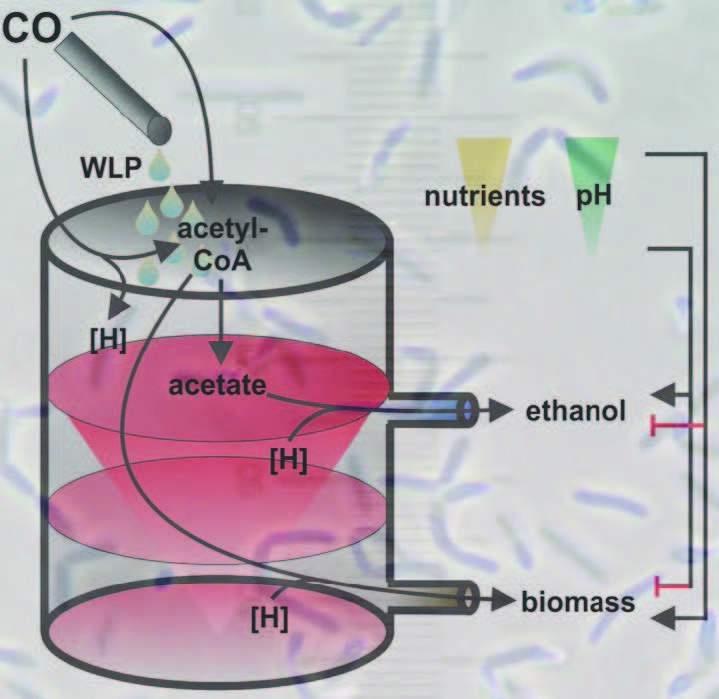 Figure 3. This model was developed to show how a thermodynamic shift can enable an anaerobic bacterium known as Clostridium ljungdahlii to use syngas fermentation to produce ethanol. (Figure courtesy of Cornell University.)
Figure 3. This model was developed to show how a thermodynamic shift can enable an anaerobic bacterium known as Clostridium ljungdahlii to use syngas fermentation to produce ethanol. (Figure courtesy of Cornell University.)
The researchers hope the details provided on the two metabolic pathways will help with optimizing syngas fermentation in the future. Additional information on this research can be found in a recent paper (
2) or by contacting Aristilde at
ludmilla@cornell.edu or Angenent at
la249@cornell.edu.
REFERENCES
1.
Canter, N. (2015), “Optimizing zeolite catalysts and sieves for base oil and biofuel production,” TLT,
71 (6), pp. 24-26.
2.
Richter, H., Molitor, B., Wei, H., Chen, W., Aristilde L. and Angenent, L. (2016), “Ethanol production in syngas-fermenting
Clostridium ljungdahlii is controlled by thermodynamics rather than by enzyme expression,”
Energy & Environmental Science,
9, pp. 2392-2399.
 Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net.