The Lubrizol Corporation
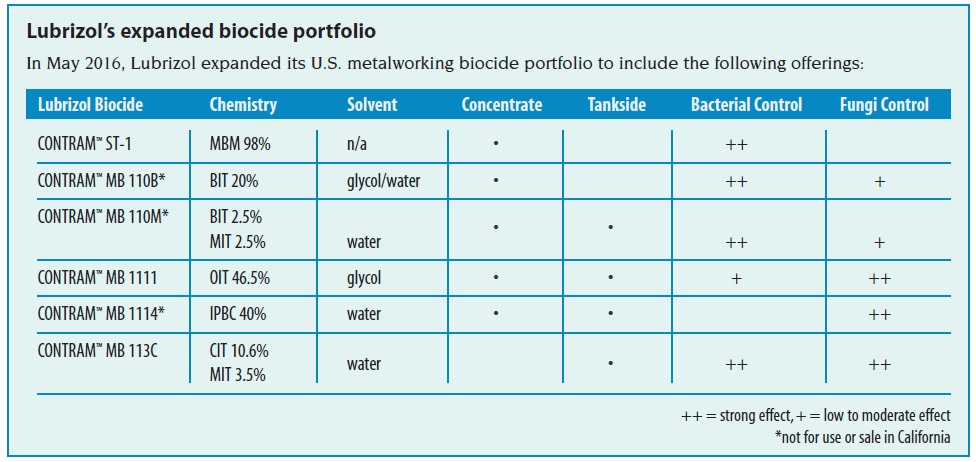
Lubrizol’s Expanded Metalworking Biocide Portfolio
By Gabe Kirsch, CMFS, North America Product Manager – Metalworking Additives | TLT CMF Plus November 2016
Water-based metal removal and metal forming operations demand complex fluids that can perform multiple functions. By nature of their ingredients, these fluids are prone to microbial growth. Metalworking biocides continue to play a key role in successful metalworking fluid (MWF) use and longevity.
A glimpse into microbes
Biocides are subdivided into bactericides (bacteria-killing) and fungicides (fungi-killing).
•
Bacteria can thrive on oxygen (aerobes), in oxygen-free environments (anaerobes), or in both conditions (facultative anaerobes)
•
Bacteria typically impact emulsifiable and semi-synthetic MWF the most
•
Fungi are usually in smaller populations than bacteria in metalworking fluids, and can congregate in splash areas
•
Synthetic fluids are usually most susceptible to fungi
In general, biogrowths may produce acids, volatile compounds, offensive odors, troublesome slime-type formations (e.g. biofilms), and/or even biotoxins. The impact of poorly-maintained metalworking fluids can be costly when bio growth is allowed to flourish. Emulsion instability, corrosion issues, clogged plumbing from growths, and “Monday morning odor” are just a few symptoms that can plague fluid systems.
Available products in the U.S.
While dozens of materials are allowed by the EPA for use as metalworking biocides, only a small number serve the vast majority of the industry’s needs. In fact, the future number of available metalworking biocides will likely dwindle. As regulatory registration costs under FIFRA remain high, only those select biocides that perform well will survive obsolescence.
Common metalworking biocide types include the following:
• Formaldehyde releasers
• Phenolics
• Quaternary ammonium compounds
• Sodium pyrithione
• Isothiazolinones
• 3-iodo-2-propynylbutylcarbamate (IPBC)
Formaldehyde releasers (FARs) include the widely-used triazine (HHT, hexahydro-1,3,5-tris(2-hydroxyethyl)-s-triazine), oxazolidines, and other materials such as MBM (N,N-methylenebismorpholine). These materials work very well against bacteria through electrophilic-nucleophilic reactions, disrupting cellular enzyme functions. However, HHT in particular has received a high degree of scrutiny due to concerns that HHT-containing fluids could lead to unacceptable formaldehyde worker exposure. The debate on use and associated hazards of FARs continues within the industry and various governments.
Phenolic biocides include ortho-phenylphenol (OPP) and p-chloro-m-cresol (PCMC). These compounds act as ‘membrane- active’ materials, interacting first with microbial cell walls to weaken their structure. Then, these biocides can enter the cells to react and eventually destroy the cells. While they are effective at controlling a variety of bacteria and fungi, phenolic compounds have periodically faced scrutiny over their environmental impact.
Quaternary ammonium compounds, such as poly[oxyethylene(dimethylimino) ethylene(dimethylimino)-ethylene dichloride] (CASN 31512-74-0), can also operate as membrane-active materials. However, this particular substance can destabilize emulsions, which limits its practical use to synthetic fluids. In addition, these ammonium-based materials in general may be deactivated by anionic compounds.
Sodium pyrithione is commonly used as a fungicide in metalworking fluids. It can react with ferric ions to create a precipitate, which unfortunately can tint fluids blue or even black through this reaction.
Isothiazolinones, which are free of formaldehyde, are a broad class of materials that act similarly to formaldehyde releasers by also following an electrophilic reaction pathway. A wide variety of derivatives are available to suit specific metalworking fluid matrices. 1,2-benzisothiazolin-3- one (BIT) is a common biocide mainly used for bacterial control in fluids. A 3:1 blend of 5-chloro-2-methyl-4-isothiazolin- 3-one (CIT) and 2-methyl-4-isothiazolin-3-one (MIT) is prevalent as a fast-acting bactericide/fungicide for tankside fluid usage. 2-n-octyl-4-isothiazolin-3-one (OIT) serves as an effective fungicide for concentrate or tankside use. Disadvantages to using isothiazolinones include lower compatibility with some amines, as well as potentially-decreasing regulatory thresholds for sensitization.
Lastly, 3-iodo-2-propynylbutylcarbamate (IPBC) is also a useful fungicide for metalworking fluids, and can be administered as a concentrate or tankside additive. When used in concentrates, it is recommended to ensure proper product stability.
 Testing and Data
Testing and Data
In order to provide representative performance data on these biocides, each was tested within its recommended treat rate range per ASTM E2275-03 (or a similar variation) in a “model” semi-synthetic fluid with the following characteristics:
•
10% mineral oil
•
MEA/TEA-TOFA buffer
•
Sulfonate emulsifier
•
Phosphate ester EP additive
•
5 v/v% pH ~9.3-9.5
For each of Lubrizol’s concentrate-registered biocides, model concentrate fluid including biocide was prepared and allowed to reside for 24 hr before 5 v/v% emulsions were made. HHT was also tested, as well as a blend of BIT and IPBC to show any possible synergy. For the tankside-only registered biocide (CIT/MIT), the model concentrate fluid without biocide was diluted to a 5 v/v% emulsion, and then dosed with this tankside biocide at the start of testing; this was done to mimic a preventive measure addition. Lastly, a blank was also tested, which was comprised of the model concentrate at 5 v/v% with no added biocide. All dilutions were made using sterile deionized water.
Three different labs were employed for ASTM testing. Below are the basic descriptions, organisms and variations that were used in each lab:
•
Lab #1: Moderate insult
o
Pseudomonas aeruginosa, Klebsiella pneumonia, Proteus mirabilis
o
Mixed fungal inoculum
•
Measurements: bacteria, fungi, pH
•
Lab #2: Aggressive insult
o
Escherichia coli, Pseudomonas aeruginosa, Pseudomonas putida, Burkholderia cepacia, Staphylococcus aureus, Alcaligenes faecalis
o
Aspergillus oryzae, Cladosporium cladosporioides, Geotrichum candidum, Paecilomyces variotti, Penicillium ochrochloron; Candida albicans, Candida guillermondi, Candida valida, Rhodotorula glutinis
•
Measurements: gram negative bacteria, yeast, mold, pH
•
Lab #3: Moderate, “adapted” insult
o
Pseudomonas putida
o
Acremonium spec.
•
Measurements: bacteria, mold
•
Special conditions for concentrates: up to four weeks of aging at up to 40°C prior to emulsion preparation and testing
Because industrial concerns still exist with hypersensitivity pneumonitis (HP), mycobacterial testing was also performed on these biocide-dosed fluids at Biosan Laboratories (Warren, MI).
Lab #1 results (
Figures 1-3) show that CONTRAM™ biocides in this specific matrix provide long-lasting control of bacteria and fungi except for CONTRAM MB 113C, which is only expected to be stable short-term as a quick kill metalworking tankside biocide. A pH decrease also occurs with CONTRAM MB 113C and the blank, which is to be expected due to concurrent biogrowth.
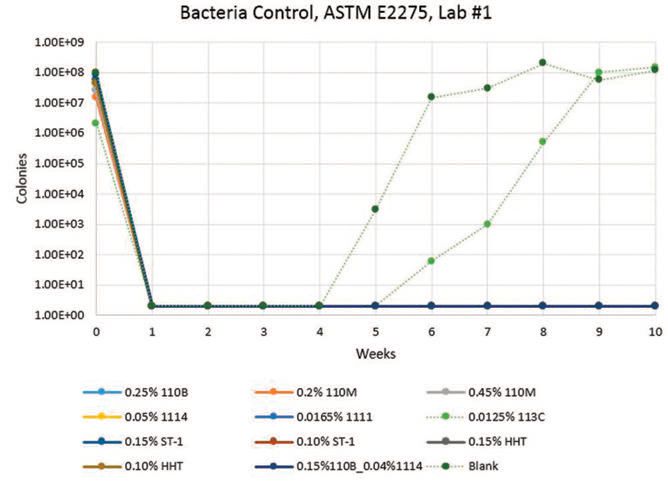 Figure 1. Lab #1, Bacteria results (solid lines represent biocides that are performing relatively well versus others in these conditions)
Figure 1. Lab #1, Bacteria results (solid lines represent biocides that are performing relatively well versus others in these conditions)
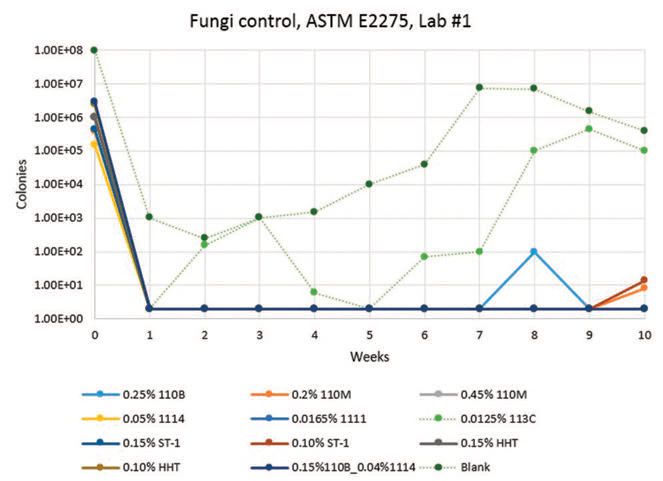 Figure 2. Lab #1, Fungi results
Figure 2. Lab #1, Fungi results
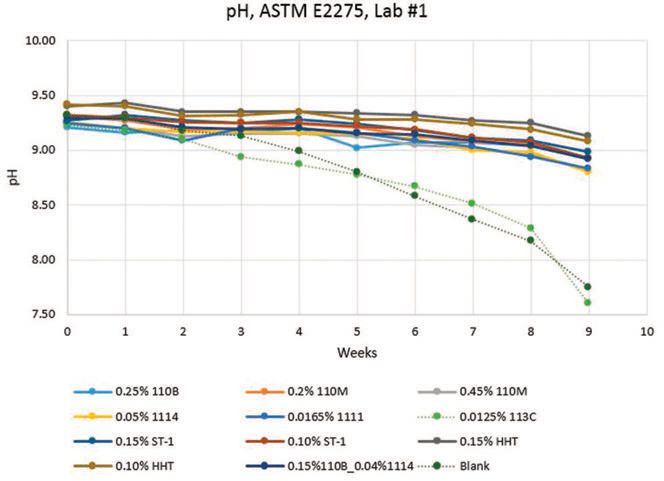 Figure 3. Lab #1, pH results
Figure 3. Lab #1, pH results
The aggressive microbial panel used in Lab #2 placed additional stress on biocides. Results generally show more differentiation between biocides in this more severe set of conditions. This supports the need for thorough, appropriate lab testing before final selection of concentrate and/or tankside products in any coolant.
Lab #3 employed a more simplified microbial broth than Lab #2. However, this study introduced aging factors (time and temperature) on biocide-formulated concentrates before emulsions were created and tested. This was done to simulate finished product storage conditions that customers may encounter. Again, some differentiation in the selected matrix was noted, but less than in Lab #2.
Mycobacterial test results also showed some ranking of biocides, with many CONTRAM biocide products providing very good control in this particular coolant matrix.
(Data for Labs #2, #3 and Biosan mycobacteria tests are not shown due to the significant number of additional charts.)
Conclusions
Microbial control is critical to maintaining the quality of metalworking fluids and their systems. Lubrizol’s CONTRAM MB isothiazolinone and IPBC biocides are well-proven chemistries for MWF microbial control. Studies show overall good performance in varying insult conditions for bacteria and fungi with our selected matrix. In addition, many biocides also help control mycobacteria in this paper’s model fluid.
Likewise, isothiazolinones and IPBC complement the popular CONTRAM ST-1. This product has extremely low formaldehyde generation versus other FAR products, and can also offer excellent biocontrol.
Depending on each metalworking fluid’s chemical matrix, operating conditions, available biodiversity, and choice of biocide, results may vary. To best select what will meet your fluid needs, pre-testing of your specific fluid and biocide per ASTM E2275 or other appropriate methods is recommended before use.
References:
Eachus, A. C. (1997). Microbial Control of Coolants. In E. R. Booser (Ed.)
Tribology Data Handbook (pp. 854-861). Boca Raton, FL: CRC Press.
Passman, F. (May 2010). Current Trends in MWF Microbicides.
TLT Tribology & Lubrication Technology, 30-38.
Passman, F. et al. (March 2016). Science vs. Fiction: MWF Biocides Part II.
TLT Tribology & Lubrication Technology, 46-57.
Paulus, W. (2005) Relationship between chemical structure and activity or mode of action of microbicides. In W. Paulus (Ed.)
Directory of Microbicides for the Protection of Materials: A Handbook (pp. 10-12). Dordrecht, The Netherlands: Kluwer Academic Publishers. 10.1007/1-4020-2818-0.
Rossmoore, H. (1995). Biocides for metalworking lubricants and hydraulicfluids. In H. W. Rossmoore (ed.)
Handbook of Biocide and Preservation Use (p. 134).
Schwingel, W.R. and Eachus, A.C. (2009). Antimicrobial Additives for Metalworking Lubricants. In L.R. Rudnick (Ed.),
Lubricant Additives: Chemistry and Applications, 2nd Edition (pp. 383-397). Boca Raton, FL: CRC Press.
Siegert, W. (2005) Microbicides for coolants. In W. Paulus (Ed.)
Directory of Microbicides for the Protection of Materials: A Handbook (pp. 215-216). Dordrecht, The Netherlands: Kluwer Academic Publishers. 10.1007/1-4020-2818-0.
Yapar, S. (2006). Removal of Phenolics from Aqueous Solutions by Using Modified Clays as Adsorbents. In R. C. Hudson (Ed.)
Hazardous Materials in the Soil and Atmosphere: Treatment, Removal and Analysis (p. 119). New York, NY: Nova Science Publishers, Inc.