Oxidation-resistant molybdenum boride
Dr. Neil Canter, Contributing Editor | TLT Tech Beat November 2016
There is good potential for MoAlB—not just as a coating but as a bulk material.
KEY CONCEPTS
•
Borides exhibit exceptional hardness properties but are vulnerable to oxidation at high temperatures.
•
A boride has now been prepared from molybdenum, boron and aluminum that displays excellent resistance to oxidation at temperatures between 1,100 C and 1,300 C.
•
This resistance to oxidation is due to the migration of aluminum from the layered structure of the boride to the surface.
BORIDES ARE TYPICALLY PREPARED FROM BORON AND A METAL. They are typically ultra-high melting point solids that display exceptional hardness in combination with good mechanical, electronic and thermal characteristics.
The primary use for borides is as metal surface coatings because their range of properties enables them to protect materials operating under such severe conditions as internal combustion engines and cutting tools.
In a past TLT article, a more efficient process for applying boride coatings onto metal surfaces was described (1). A very dense, iron (III) boride coating that has a thickness over 90 microns can be applied in only 30 minutes at a temperature ranging between 770 C and 950 C.
This much more energy efficient and environmentally friendly process is known as ultra-fast boriding. Coating 9310 gear steel using this process led to superior coefficient of friction and wear reduction compared to carburized steel.
But use of borides is restricted due to their tendency to readily oxidize at high temperatures. Michel W. Barsoum, Distinguished professor of materials science and engineering at Drexel University in Philadelphia, says, “Borides operate very effectively at high temperatures (defined as temperatures above 1,000 C) and can provide a protective coating to metals. But borides are vulnerable to oxidation when placed in an oxygen-rich, high-temperature environment. This makes them unsuitable for applications such as hypersonic flight and jet engine turbine blades.”
Barsoum believes that if a process can be developed to make borides more oxidation resistant, then their use will expand. One approach is to develop boride structures similar to the layered early transition metal carbides and nitrides known as MAX phases. Barsoum says, “Approximately 20 years ago we developed MAX, ternary carbides and nitrides that when prepared with the correct metals—mainly aluminum—were quite oxidation resistant at high temperatures. The use of aluminum in a MAX leads to the formation of an aluminum oxide coating that can resist further oxidation at high temperatures.”
An appeal in working with a MAX type product is that using more than two elements will increase the chances for producing a material with unique properties. Barsoum explains, “Adding at least a third element can sometimes lead to a layered structure that can enhance the characteristics of the binary material. For example, titanium carbide and silicon carbide are both hard, non-machinable materials. However, when titanium, silicon and carbon are combined, this produces a layered material—Ti3SiC2—while still hard relative to metals, is soft enough to be machined with a manual hack saw.”
Use of this strategy has now led to the development of a new boride that exhibits greater oxidation resistance at high temperatures.
DEFICIENCY OF ALUMINUM
Barsoum and his colleagues now report the synthesis of a layered boride from molybdenum, boron and aluminum, MoAlB. The compound is formed through hot-pressing the reactants in a graphite foil-lined cylindrical graphite die heated to a temperature of 1,200 C at a rate of 300 C per hour. Once the temperature is reached, the reaction mixture is subjected to a pressure of 39 megapascals for nearly seven hours.
Barsoum says, “High-resolution scanning transmission electron microscopy shows that MoAlB contains two aluminum layers sandwiched in between a molybdenum boride sub-lattice (see Figure 2). This layered structure is reminiscent of the MAX structure.”
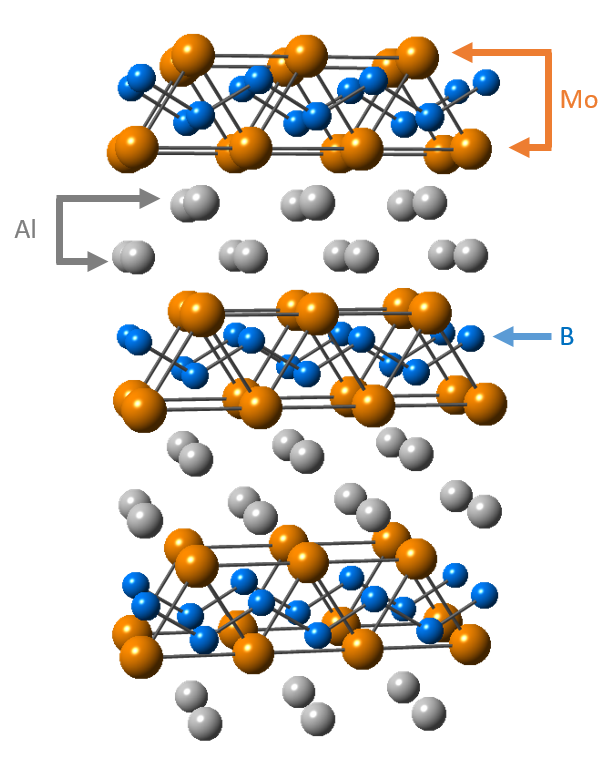
Figure 2. The layered structure of MoAlB enables aluminum atoms (gray) to migrate to the surface, enhancing the oxidation resistance of the material at high temperatures. (Figure courtesy of Drexel University.)
When oxidized at 1,100 C and 1,300 C for at least 100 hours, MoAlB displayed excellent resistance to oxidation. The researchers observe the formation of dense, adherent aluminum oxide scales on the surface of the material that protects the material from further oxidation. Analysis of the scale indicates that aluminum diffuses out of the MoAlB during oxidation and forms a layer of aluminum oxide or alumina. This means that MoAlB can exist with a small deficiency of aluminum at elevated temperatures.
Barsoum says, “The structure of MoAlB becomes unstable as it is heated facilitating the migration of aluminum from the layered structure to the surface. This effect can happen because only a small fraction of the aluminum forms coatings that can range in thickness from 3 to 20 microns. But the stoichiometry of MoAlB can support this loss up to a temperature of 1,400 C.”
Barsoum does indicate that if a high enough temperature is reached under vacuum conditions, sufficient aluminum will leave the material forcing a conversion back to molybdenum boride. He also points out that the oxidation properties of MoAlB are vastly superior to zirconium diboride, which is used widely in high-temperature aerospace applications.
The layering in MoAlB also accounts for the material’s reduced hardness compared to other materials. Barsoum says, “The aluminum layer present in MoAlB imparts some ductility to the material enabling it to be softer.”
This combination of excellent resistance to oxidation at high temperatures means there is good potential for MoAlB as not just a coating but as a bulk material. Barsoum says, “In the future, we will examine the mechanical properties of MoAlB, including how the material performs when it loses aluminum at high temperatures.”
Additional information can be found in a recent article (2) or by contacting Barsoum at barsoumw@drexel.edu.
REFERENCES
1. Canter, N. (2013), “More efficient surface treatment,” TLT, 69 (2), pp. 8-9.
2. Kota, S., Solvas, E., Ly, A., Lu, J., Elkassabany, O., Huon, A., Lee, W., Hultman, L., May, S. and Barsoum, M. (2016), “Synthesis and characterization of an alumina forming nanolaminated boride: MoAlB,” Scientific Reports, 6, Article Number: 26475.
 Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat can be submitted to him at neilcanter@comcast.net
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat can be submitted to him at neilcanter@comcast.net.