Sodium polyhydrides: Potential superconductors
Dr. Neil Canter, Contributing Editor | TLT Tech Beat November 2016
Compounds with multiple hydrogens have been prepared for the first time.
KEY CONCEPTS
•
Development of a superconductive material at room temperature may be achieved through working with metallic hydrogen.
•
Polyhydrides (compounds containing multiple hydrogen atoms) have been synthesized for the first time under extreme experimental conditions.
•
Polyhydrides may be useful as precursors to hydrogen-based superconductors.
DEVELOPMENT OF A MATERIAL that exhibits superconductivity at room temperature continues to be a long-term research goal. The reason is that superconductors can conduct electricity without causing any resistance. As a result, no energy is dissipated as heat, and friction is not observed.
One material that exhibits superconductivity closest to room temperature is cuprates or copper-oxide ceramics. In a previous TLT article, a technique known as resonant soft x-ray scattering was used to identify a phenomenon known as electronic nematicity, which represents a breakdown in the rotational symmetry of these materials (1). The researchers speculate that electronic nematicity may facilitate the onset of superconductivity at temperatures closer to room temperature.
One approach to produce superconductors is to work with metallic hydrogen. Dr. Viktor Struzhkin, team leader in the Geophysics Laboratory of the Carnegie Institution of Washington in Washington, D.C., says, “The hydrogen molecule has been known to exist as a molecular solid for many years. Due to its position as an alkali metal on the periodic table, theoretical predictions have been made that under high pressures, hydrogen can form a metallic phase at enormous pressures about 500 GPa (5 million atmospheres). This conversion may lead to the formation of metastable materials at lower pressures, which would be composed of hydrogen chains or filaments where atoms of hydrogen are linked and electrons are now free to flow through the material.”
Struzhkin points out that metallic hydrogen and room temperature superconductors have never been found in nature. One approach to facilitate the formation of metallic hydrogen is to work with compounds that contain multiple hydrogen atoms.
Theoretical analysis has shown that compounds with multiple hydrogens can be prepared through reaction of a hydrogen rich alloy with another alkali metal. Formation of such a species may facilitate the preparation of a hydrogen-based superconductor. Such a species known as a polyhydride has now been synthesized for the first time.
DIAMOND ANVIL CELL
Struzhkin and his colleagues have for the first time synthesized compounds with multiple hydrogens that are known as sodium polyhydrides. The experiments are done through the use of a laser-heated diamond anvil cell under extreme temperature and pressure conditions.
Sodium hydride, sodium and a small amount of gold particles are charged into a diamond anvil cell in a controlled atmosphere glove box (less than 1 ppm of oxygen present). Hydrogen gas is then added until a pressure of 200 megapascals is reached. Struzhkin says, “Sodium hydride is a transparent material that cannot couple the heating benefits of the laser. We use gold particles as a means to enable the laser to increase the temperature to the level where we might be able to detect a reaction.”
Once the experimental conditions reached 2,000 K at pressures between 30 and 40 gigapascals, the researchers detected a hot flash indicative of a reaction. Struzhkin says, “When a reaction is induced under high pressure laser experiments, excessive heat is released in an exothermic process that appears as a bright flash.”
The researchers estimated that the exotherm caused the temperature of the process to increase to between 4,000 K and 6,000 K. Another indication of a reaction is that the sample present in the diamond anvil cell changes shape. Under these conditions, the researchers decided not to subject the diamond anvil cell to further heating due to the risk of breaking the apparatus.
Analysis of the products generated by the reaction is conducted through the use of x-ray diffraction and Raman spectroscopy. Struzhkin says, “We needed to do the analysis of the reaction mixture under at least 18 gigapascals of pressure. Below this pressure, the products are not stable.”
Analysis of the x-ray diffraction patterns shows the presence of a crystal structure containing sodium polyhydride species. The major component in the product is identified as a sodium polyhydride with a NaH3 stoichiometry. But data also indicates that a species with a larger number of hydrogen atoms (NaH7) also is present as are potentially higher polyhydrides containing more than seven hydrogen atoms.
The Raman spectrum of the product mixture suggests that hydrogen molecules are present within the sodium polyhydride crystal structure. Theoretical analysis supports this experimental data as the researchers find that the most stable polyhydride composition is (NaH)m(H2)n where m and n are integers.
Struzhkin says, “We believe that under the severe experimental conditions, a hydrogen molecule will react with a hydride anion to form a H3 linear anion configuration.” Figure 1 shows a schematic of the possible structure of the polyhydride, NaH7 with H3 species surrounded by hydrogen atoms.
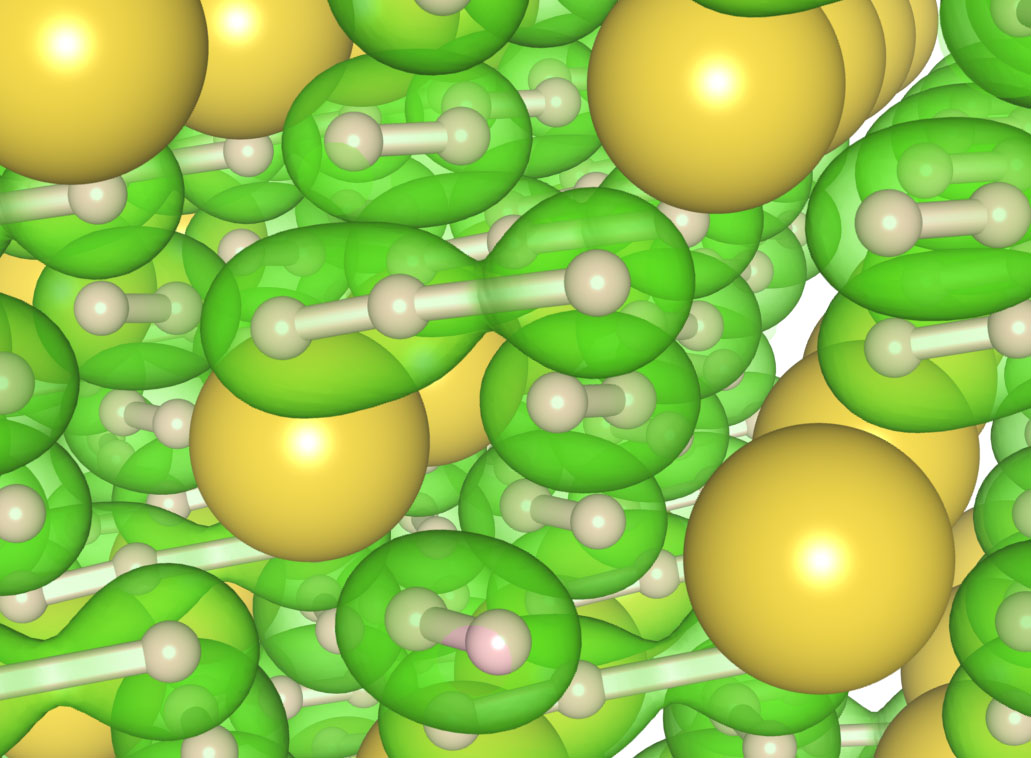
Figure 1. Depicted is a schematic of NaH7, one of the polyhydrides synthesized for the first time. A new three-atom hydrogen chain shown in green in the center of the figure is surrounded by two-atom hydrogen molecules, also shown in green. (Figure courtesy of Carnegie Institution of Washington.)
Future work will involve trying to synthesize polyhydrides with other cations. Struzhkin says, “An attempt will be made to synthesize cesium polyhydrides because they are theoretically supposed to be metastable at lower pressures (1 gigapascal) than sodium polyhydrides. But cesium is more reactive than sodium, which will make this effort more challenging.”
Struzhkin believes that the identification of sodium polyhydrides is a significant step forward in developing a room temperature superconducting material. He says, “One idea to produce a superconducting material is to dilute the polyhydride with hydrogen to create a phase that is rich in H2. Ultimately, efforts need to continue to find the right polyhydride.”
Additional information can be found in a recent article (2) or by contacting Struzhkin at vstruzhkin@carnegiescience.edu.
REFERENCES
1. Canter, N. (2016), “Potential nematicity to effect superconductivity,” TLT, 72 (5), pp. 18-20.
2. Struzhkin, V., Kim, D., Stavrou, E., Muramatsu, T., Mao, H., Pickard, C., Needs, R., Prakapenka, V. and Goncharov, A. (2016), “Synthesis of sodium polyhydrides at high pressures,” Nature Communications, 7, Article Number: 12267.

Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat can be submitted to him at neilcanter@comcast.net.