Lithium-Ion Battery Anode From Fungus
Dr. Neil Canter, Contributing Editor | TLT Tech Beat July 2016
The new technology could lead to longer battery life and reduced flammability.
KEY CONCEPTS
•
Carbon fibers obtained from the wild fungus
Tyromyces Fissilis have been incorporated into the anode of a lithium-ion battery.
•
During the processing, carbonization is conducted at 500 C so that the resulting carbon fibers are more disordered and have a larger cavity.
•
Cobalt oxide nanoparticles are included to boost performance of the hybrid anode to one and a half times greater than graphite.
LITHIUM-ION BATTERIES continue to be the preferred rechargeable energy option, but progress in using them has been hindered due to longevity and flammability problems. Part of the cause has been traced to the anode, which is usually prepared from graphite.
A past TLT article details how metal filaments known as dendrites can form at a graphite anode from fibers of lithium or other metals leading to the generation of a network of fern-like structures that if unchecked will short circuit the battery leading potentially to catastrophic failure (
1).
One way to correct this problem is to develop an all-solid-state lithium-ion battery. Such a battery based on lithium, germanium, phosphorus and sulfur has been developed (
2). A specific compound based on these four elements acts as the anode, cathode and the electrolyte. Initial results were promising, but the battery did not perform as well as one using a liquid electrolyte.
Vilas Pol, associate professor of chemical engineering at Purdue University in West Lafayette, Ind., says, “The biggest problem with lithium-ion batteries is that they are limited in their energy density and power output which means that faster cycling rates that involve charging and discharging are not possible.”
Pol believes that other anode options besides graphite should be evaluated because graphite only allows lithiation (battery charge) at low operating voltages leading to the possibility of lithium plating which can facilitate battery failure. He says, “Graphite’s layered structure is a concern because lithium can plate out on the graphitic surface.”
A preferred approach is to evaluate amorphous materials for use as anodes. Pol says, “Carbon fibers are one promising option because they contain disordered carbon structures that in contrast to graphite facilitate faster electron hopping leading to the faster charge/discharge cycles needed to boost lithium-ion battery performance. The reason for this boost in performance is because carbon fibers are one-dimensional structures and have larger inter-layer spacing than graphite.”
Finding a low-cost, readily available source of carbon fibers would be desirable in preparing more cost-effective lithium-ion batteries. Such a source has now been found and shows promise when processed into anodes.
TYROMYCES FISSILIS
Pol and doctoral student Jialiang Tang have incorporated carbon fibers from the wild fungus
Tyromyces Fissilis (TF) into lithium-ion battery anodes that demonstrate superior performance to graphite. Pol says, “We selected TF because one of our research objectives is to learn from nature. TF is a mushroom that exhibits a fiber-like architecture and is readily available in nature as it grows throughout the U.S. TF has a high concentration of fibers compared to other fungi which can form a conductive, interconnected network. This fungus is also not edible, so we are not interfering with a food source.”
The researchers obtained TF samples from oak tree trunks and extracted carbon fibers in what Pol terms a straight-forward process. He says, “Samples of TF are cleaned with water to remove surface debris and then vacuum-dried at 80 C overnight. The resulting material can then be either used as is or sliced into smaller pieces.”
The next step in the process is to carbonize the material to produce carbon fiber samples. Pol says, “Carbonization is conducted at high temperatures and care must be taken to ensure that carbon monoxide and carbon dioxide, the ultimate decomposition products, are not produced. We conducted the pyrolysis under an inert atmosphere using argon to prepare the carbon fibers.”
Figure 2 shows microscopic images of the fiber network from a dried slice of TF on the left and the same network after carbonization on the right.
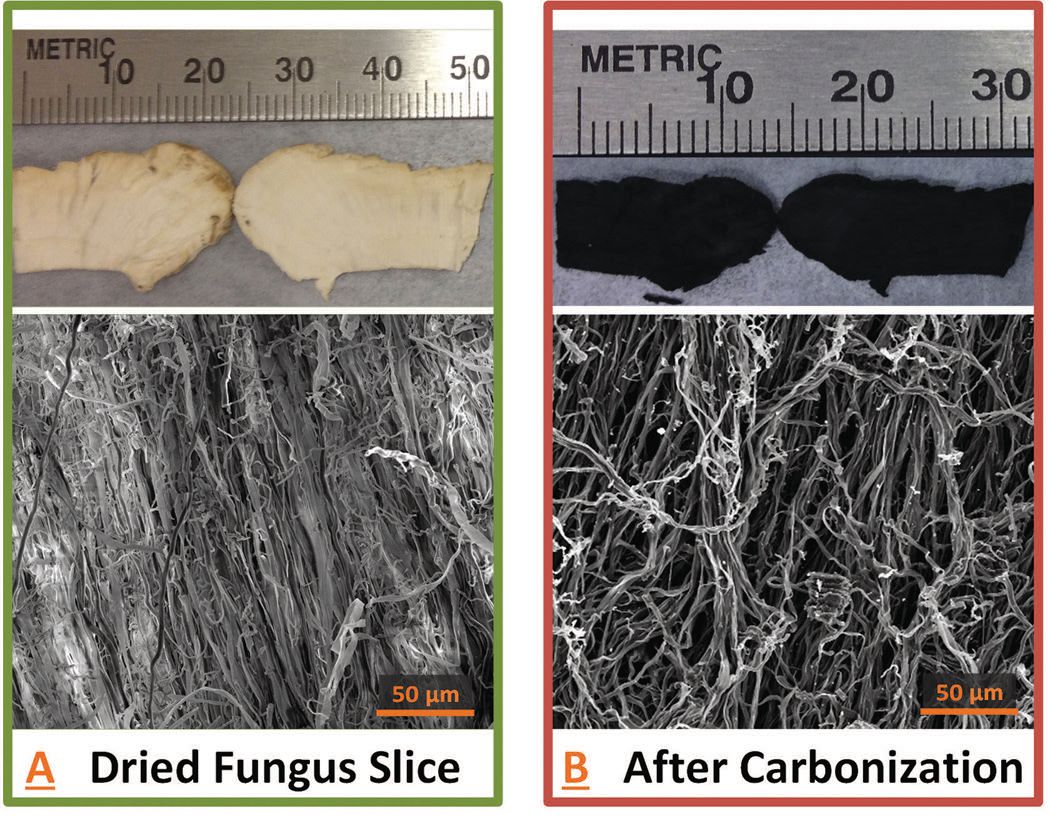 Figure 2. Carbonization at high temperatures is used to convert a dried slice of TF on the left to an interconnected network of fibers suitable for use in a battery anode on the right. (Figure courtesy of Purdue University.)
Figure 2. Carbonization at high temperatures is used to convert a dried slice of TF on the left to an interconnected network of fibers suitable for use in a battery anode on the right. (Figure courtesy of Purdue University.)
Carbon fiber is carbonized at three temperatures (500 C, 800 C and 900 C) for three hours to determine the effect of temperature on performance. Pol says, “A lithium-ion battery anode is prepared by mixing 80% of the TF derived carbon fiber with 10% carbon black and adding 10% polyvinylidene fluoride as a binder. N-methylpyrolidine is used as a solvent to facilitate homogenization. The resulting slurry is coated on a copper current collector, dried and punched into 12-millimeter diameter disk electrodes.”
Charge/discharge cycles of a battery prepared with the TF derived carbon fiber anode shows better performance for material carbonized at the lowest temperature, 500 C. Pol says, “The structure of the carbon fiber produced at 500 C is more disordered than what is found at higher temperatures, enabling more lithium ions to be contained in the anode. Carbon fiber produced at the higher temperature has a smaller cavity and exhibits a structure close to graphite where lithium ions can plate on the surface of graphite.”
To boost the performance of carbon fiber, the researchers added cobalt oxide nanoparticles with average diameters below 50 nanometers through a solid-state thermal process. Pol says, “Both carbon fiber and cobalt oxide are electrochemically active and by combining them, we produce a synergistic effect that leads to a hybrid anode with a stable capacity that is one and a half times greater than graphite.”
The researchers dosed the carbon fiber with 1 mol % and 10 mol % cobalt oxide per one mole of the hybrid material. Superior performance was found at the higher mol loading percentage.
Future work will involve testing of the anode hybrid to minimize first-cycle capacity loss during cycling and to evaluate the material in a real lithiumion battery. Additional information can be found in a recent article (
3) or by contacting Pol at
vpol@purdue.edu.
REFERENCES
1.
Canter. N. (2015), “Dendrite-suppressing battery technology,”
Tribology and Lubrication Technology,
71 (4), pp 14 – 15.
2.
Canter. N. (2015), “Single-material lithium-ion battery,”
Tribology and Lubrication Technology,
71 (8), pp 14 – 16.
3.
Tang, J., Etacheri, V. and Pol, V. (2016), “Wild Fungus Derived Carbon Fibers and Hybrids as Anodes for Lithium-ion Batteries,”
4 (5), pp 2624 – 2631.
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat can be submitted to him at neilcanter@comcast.net.