New single-layer two-dimensional material
Dr. Neil Canter, Contributing Editor | TLT Tech Beat June 2016
A silicon boron nitrate material has the potential to overcome the limitations of graphene.
KEY CONCEPTS
•
A theoretical analysis identifies a new, stable two-dimensional material that contains boron, nitrogen and silicon atoms.
•
Analysis of the theoretical material shows that it is metallic and thermally stable up to 1200 K.
•
Research is underway to synthesize this silicon boron nitride material in the laboratory.
EXTENSIVE RESEARCH HAS BEEN CONDUCTED on the two-dimensional arrangement of single-layer carbon atoms arranged in hexagonal structures and known as graphene. This material has been the subject of a number of articles in this column due to its potential for use as a solid lubricant.
In a past TLT article, researchers determined that graphene is the strongest material ever examined and exhibits a break strength 200 times greater than the strength of steel (
1). A second TLT article details how difficulties in dispersing graphene in hydrocarbon base stocks were overcome through a crumpling process that involves starting with a derivative, graphene oxide (
2). Crumpled graphene balls display better dispersion stability in polyalphaolefin leading to superior friction and wear results even compared to a commercially available 5W-30 engine oil.
Dr. Madhu Menon, associate director of the Center for Computational Sciences at the University of Kentucky in Lexington, Ky., says, “Graphene is an excellent material to work with as this material demonstrates outstanding performance properties and can be used in many applications.”
But graphene does have performance limitations. Menon says, “Graphene is metallic in nature and does not have the ability to perform as a semiconductor because no gap exists between the valence band of this material and the conduction band. Functionalization of graphene is also difficult to achieve because carbon atoms prefer to be bonded to three other atoms, which is the case with this material. The easy way to prepare derivatives is by using graphene’s edges. A final limitation is that from a size perspective, carbon atoms cannot be easily displaced in graphene with a different atom such as a metal.”
In an effort to identify materials with better performance characteristics, researchers started to evaluate molybdenum disulfide and transitionmetal dichalcogenides. Menon says, “The problem with these materials is that they contain three-atom thick structures, are heavier than graphene and much more expensive.”
In searching for potentially a more cost-effective two-dimensional material that is also truly flat, Menon contends that researchers have not spent much time evaluating atoms present in the first and second rows of the Periodic Table. Carbon boron nitride was evaluated but is not satisfactory. Menon says, “Carbon boron nitride forms a bubbly, three-dimensional structure and not a two-dimensional structure. A second negative is that the carbon atoms are segregated from boron nitride in specific blocks within the material.”
New research has just been disclosed detailing the potential existence of a two-dimensional material that appears to be stable based on model simulation studies.
SILICON BORON NITRIDE
Menon and his fellow researchers evaluated combinations of the atoms boron, nitrogen and silicon theoretically to determine if any specific stoichiometry will produce a two-dimensional material that is also flat. He says, “We decided to introduce silicone atoms into a boron nitride matrix and evaluated all perturbations that will generate a two-dimensional hexagonal structure. After extensive theoretical computations, we found that only one planar structure that combines the three atoms is stable.”
The structure is shown in Figure 2 and has a Si
2BN base structure where each silicon atom has a second silicon, one boron and one nitrogen atom as nearest neighbors. Menon says, “Our goal is to find a material that has a sp
2 planar bonding orientation that also contains substantial double bond characteristics. Boron nitride forms strong double bonds and has a planar sp
2 structure. Even though silicon prefers to be in a sp
3 orientation, we are able to identify a two-dimensional silicon boron nitride material that contains both sp
2 bonds and a large number of double bonds.”
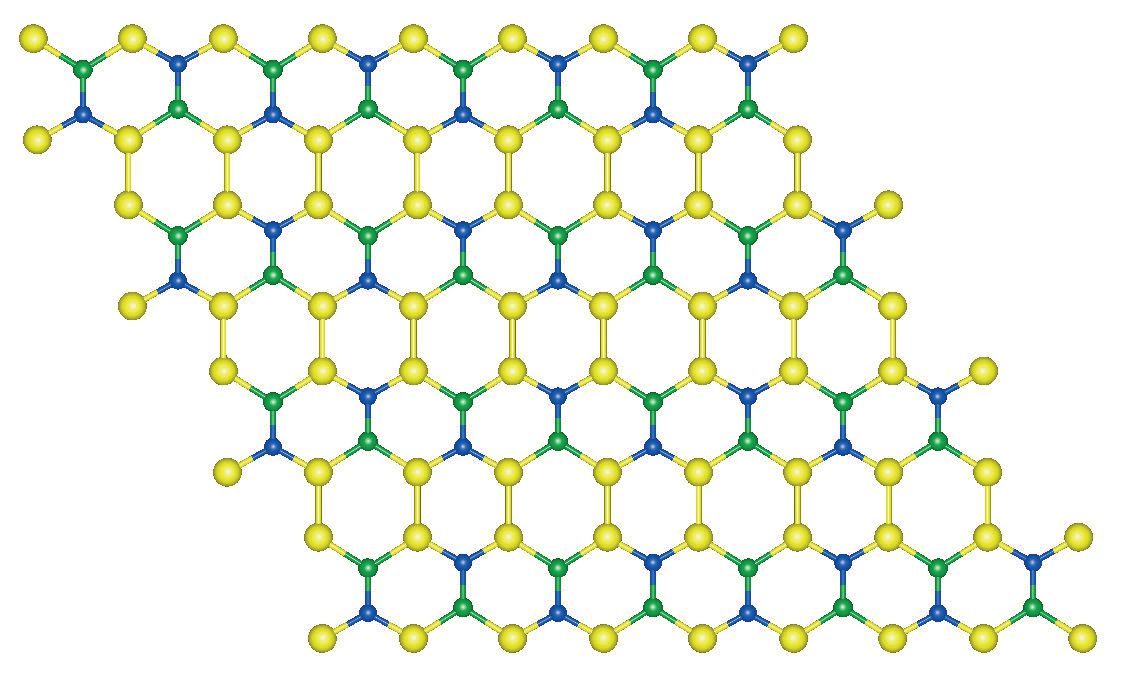 Figure 2. A new silicon boron nitride material has been identified through theoretical analysis to display the two-dimensional planar structure shown. (Figure courtesy of the University of Kentucky.)
Figure 2. A new silicon boron nitride material has been identified through theoretical analysis to display the two-dimensional planar structure shown. (Figure courtesy of the University of Kentucky.)
The vibrational frequencies of the new silicon boron nitride material are calculated to determine if it is stable. As part of this process, the phonon frequencies obtained are all found to be real. If any had been imaginary, then it would indicate structural instability.
Thermal stability analysis is done by running simulations where the silicon boron nitride material is heated to 1200 K. Menon says, “After cooling the material at the end of the simulations, we found that it returned to its original structure signifying that no bonds are broken and the structure is stable.”
Fermi energy analysis of the electron density in the silicon boron nitride material shows that it is metallic. Menon envisions that nanotubes can be prepared from this material in a similar manner to graphene. He adds, “The difference is that the nanotubes will not be symmetrical because the bond lengths in the silicon boron nitride are different as compared to graphene. Simulations show that the nanotubes are quite stable.”
Menon believes that the silicon boron nitride material can be easily derivatized through addition of other atoms such as hydrogen. He says, “Derivatives can be readily added to the silicon atoms creating pyramids above the single-atom layer. In the case of hydrogen, the resulting material will function with the properties of a semiconductor.”
One other intriguing aspect of the silicon boron nitride material is the possibility of substituting metals in the structure for silicon. Menon says, “In contrast to graphene, silicon atoms are much larger, and this offers the opportunity to substitute metal atoms for them in derivatives that could lead to new materials with interesting properties.”
Currently, Menon is collaborating with researchers to determine if the silicon boron nitride material can be synthesized. You can find additional information on this research in a recent article (
3) or by contacting Menon at
madhu@ccs.uky.edu.
REFERENCES
1.
Canter, N. (2009), “Graphene: The strongest material ever examined,”
Tribology and Lubrication Technology,
65 (2) pp 28 – 29.
2.
Canter, N. (2016), “Evaluation of a new lubricant additives: Crumpled graphene balls,”
Tribology and Lubrication Technology,
72 (4), pp 12 – 13.
3.
Andriotis A., Richter, E. and Menon, M. (2016), “Prediction of a new graphenelike Si2BN solid,”
Physical Review B,
93 (8), 081413 (R).
 Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net.