New alloy for low-temperature applications
Dr. Neil Canter, Contributing Editor | TLT Tech Beat April 2016
This material works well at low temperatures and exhibits higher levels of toughness and strength as the temperature drops.
KEY CONCEPTS
•
A high-entropy alloy prepared with equal parts of chromium, manganese, iron, cobalt and nickel shows excellent toughness at low temperatures.
•
The performance of this alloy at low temperatures is due to a combination of slow-moving and fast-moving dislocations that work synergistically.
•
The researchers hope to develop a strategy for systematically evaluating the large number of high-entropy alloys not yet under evaluation.
FINDING METAL ALLOYS THAT HAVE GREATER TOLERANCE TO DAMAGE is a longstanding objective of researchers. Two factors considered are evaluating an alloy’s resistance to deformation (strength) and resistance to fracture (toughness).
Traditional alloys have been produced by mixing a dominant element with small percentages of secondary components that can upgrade performance in certain areas. For example, adding carbon to iron produces a steel alloy that is stronger. Better corrosion resistance is achieved by mixing iron with chromium and nickel.
In the search for better performing materials, researchers turned to mixing more than two elements together, but led to the formation of metallic glasses. Robert Ritchie, H.T. & Jessie Chua Distinguished Professor of Engineering in the department of materials science & engineering at the University of California, and faculty senior scientist, material sciences division of Lawrence Berkeley National Laboratory in Berkeley, Calif., says, “The effort in combining five to six elements together leads to sufficient ‘confusion’ in the solidification of the alloy that the end result is a glass as opposed to a polycrystalline material.”
Interestingly, further research has found that putting five or more elements together can lead to the formation of a single-phase crystalline material that is known as a high-entropy alloy. In a past TLT article, the preparation of such an alloy based on mixing equal percentages of aluminum, magnesium, scandium and titanium was discussed (
1). The researchers claim that at the time of their work, this alloy exhibits a higher strength-to-weight ratio than any other existing metal.
Ritchie says, “High-performance alloys that exhibit high levels of toughness and strength combined with good ductility at low temperatures have been hard to identify. The reason is that as the temperature drops, most metals will lose ductility and become more brittle.”
With the growing use of natural gas, there is a need for better constructed tanks to handle liquefied natural gas. Ritchie says, “Exploring the many potential combinations of high-entropy alloys is a promising direction for us because this alloy type has the potential to exhibit an exceptional combination of strength and toughness.”
Determination of the mechanical properties of a high-entropy alloy has now found a material that not only works well at low temperatures but exhibits higher levels of toughness and strength as the temperature drops.
SYNERGISM OF MECHANISMS
Ritchie and his collaborators—who studied the mechanical properties of a high-entropy alloy containing equal percentages of chromium, manganese, iron, cobalt and nickel—found that the alloy is present in a single-phase, face-centered cubic structure and demonstrates exceptional properties, particularly at low temperatures. He says, “This alloy has been known for about 10 years, but very little was done to study its mechanical properties prior to fairly recently. We find that the toughness of this alloy exhibits numbers that are off the chart and are comparable to the very best cryogenic steels currently used, which are austenitic stainless steels and high-nickel containing steels.”
The toughness value that the researchers obtained exceeds 200 megapascals-m
1/2. This figure remained relatively constant when measured at temperatures ranging from room temperature (293 K) down to 77 K. Ritchie says, “At this time, the lowest temperature we can measure at is 77 K, which is the boiling point of liquid nitrogen. We are hoping in the future to determine how to measure the toughness of this alloy at the boiling point of liquid helium, which is 4 K.”
The alloy also exhibits excellent tensile strength values in the range of 1 gigapascal that increase by at least 70% as the temperature drops from 293 K to 77K. Tensile ductility also increases over that temperature range by approximately 25%.
The researchers next turned to transmission electron microscopy (TEM) to determine what makes this high-entropy alloy so special. Ritchie says, “Data from TEM shows that a combination of slow-moving and fast-moving dislocations (or defects in the metal crystal) work synergistically to enable the high-entropy alloy to exhibit excellent toughness, strength and ductility at low temperatures. Some dislocations move slowly while others occur early to enable the material to exhibit exceptional ductility at low temperatures.”
Figure 3 shows TEM images of the slow planar slip of dislocations going through the high-entropy alloy. However, Ritchie and his co-workers observe that at cryogenic temperatures, the preferred deformation mode for this alloy is twinning where the atomic arrangements in adjacent crystalline regions form mirror images of one another. He says, “This mechanism is even more potent for inducing strength and ductility.”
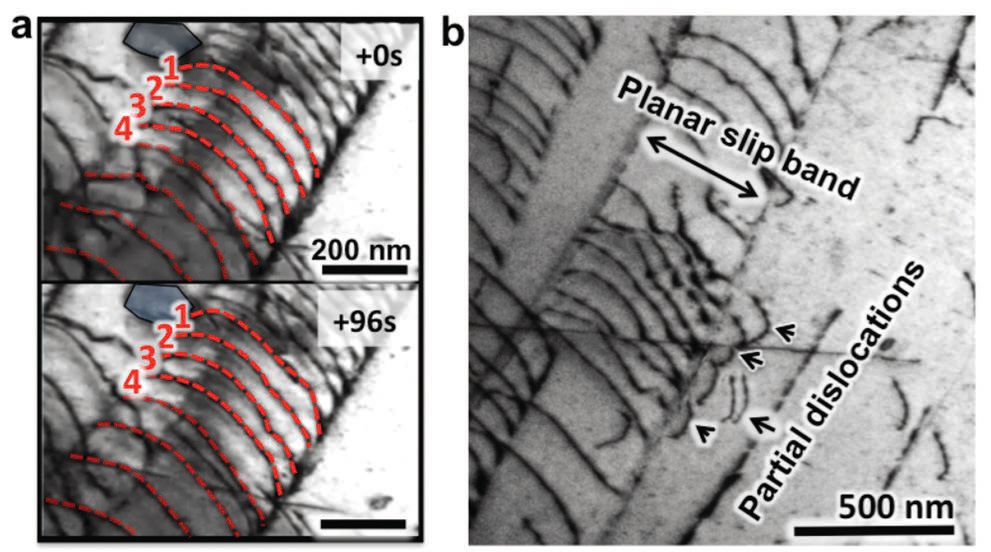 Figure 3. A high-entropy alloy displays outstanding properties at low temperatures due to a synergism between fast-moving dislocations and the slow planar slip of dislocations shown. (Figure courtesy of the University of California and the Lawrence Berkeley National Laboratory.)
Figure 3. A high-entropy alloy displays outstanding properties at low temperatures due to a synergism between fast-moving dislocations and the slow planar slip of dislocations shown. (Figure courtesy of the University of California and the Lawrence Berkeley National Laboratory.)
Another unusual aspect of this high-entropy alloy is that its toughness is due to both intrinsic toughening and extrinsic toughening mechanisms. Ritchie says, “Intrinsic mechanisms operate in advance of a crack tip to provide resistance to microstructural damage while extrinsic mechanisms act behind the crack to prevent it from growing within the metal crystal.”
Further work needs to be done to commercialize this alloy, which could take decades, according to Ritchie. He says, “Very little is known about what other high-entropy alloys may exist and have exciting, perhaps unprecedented properties. They represent a ‘black hole,’ and nobody knows yet what is out there. We hope to develop the right scientific strategy in the future for systematically examining this vast spectrum of unexplored materials.”
Additional information can be found in two recent articles (
2, 3) and by contacting Ritchie at
roritchie@lbl.gov.
REFERENCES
1.
Canter, N. (2015), “High-entropy alloys,” TLT,
71 (3), pp. 14-15.
2.
Gludovatz, B., Hohenwarter, A., Catoor, D., Chang, E., George, E. and Ritchie, R. (2014), “A fracture-resistant high entropy alloy for cryogenic applications,”
Science,
345 (6201), pp. 1153-1158.
3.
Zhang, Z., Mao, M., Wang, J., Gludovatz, B., Shang, Z., Mao, S., George, E., Yu, Q. and Ritchie, R. (2015), “Nanoscale origins of the damage tolerance of the high-entropy alloy CrMnFeCoNi,
Nature Communications 6, 10143, DOI: 10.1038/ncomms10143.
 Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net.