Cast iron alloys: Better analysis approach
Dr. Neil Canter, Contributing Editor | TLT Tech Beat March 2016
Researchers are using synchrotron x-ray analysis to develop three-dimensional images.
KEY CONCEPTS
•
Though widely used in many applications, cast iron is still prepared by a combination of art and science.
•
Synchrotron x-ray analysis is now being used to produce a three-dimensional image of cast iron alloys.
•
This technique should help users better correlate the microstructure of cast iron with its properties.
THE CONTINUING EFFORT TO IMPROVE THE PERFORMANCE AND EFFICIENCY of machinery is leading researchers to develop new lightweight alloys and also techniques for better understanding how the structure of these alloys correlate with their physical and mechanical properties and ultimately their performance. For example, much attention has been paid to working with non-ferrous alloys in automobiles in an effort to improve their fuel economy.
A previous TLT article discussed how the presence of oxygen in titanium causes the metal’s physical properties to decline (
1). Typically other components known as solutes are added to metals to improve their properties. In the case of titanium, researchers found that oxygen can accelerate the formation of extra spaces in the metal lattice known as dislocations. This phenomenon causes tangling leading the titanium to become more vulnerable to cracking.
A class of metal alloys that continues to be widely used in many applications in the energy, manufacturing and transportation industries is cast iron. This metal has been used since the 17th Century through a casting process, which involves the use of molten iron and solutes including carbon, silicon, manganese, sulfur and phosphorus. Carbon in particular is a very important solute because its presence will improve the alloy’s hardness and strength but too much will increase the brittleness of cast iron.
Dr. Dileep Singh, group leader of thermal-mechanical research at Argonne National Laboratory’s Center for Transportation Research in Argonne, Ill., says, “The amount of carbon added to iron in the preparation of cast iron controls fatigue, strength and thermal properties. The size and morphology of the graphite phase, resulting from carbon additions, in cast iron has been found to be very important in determining the properties of the alloy formed.”
An important aspect of cast iron preparation is inoculation in which various metals are added to the casting melt, which affects the process of solidification and greatly influences the final structure and properties of the alloy. Singh says, “By adjusting the composition of the solutes and the type of inoculants, one can tune the mechanical and physical properties of the cast iron in order to optimize the final product. But achieving the desired result is a combination of art and science.”
Making the process more difficult is that the typical method for examining the microstructure of cast iron is using two-dimensional optical microscopy or scanning electron microscopy imaging. Singh says, “The problem with the two-dimensional analysis is that examination of a cross-section of the cast iron does not reveal complete information about the shape and size of the graphite particles formed when the alloy is produced.”
A new technique is needed to analyze cast iron in order to better correlate its microstructure with its properties. Such a technique now has been developed.
SYNCHROTRON X-RAY ANALYSIS
Singh and his colleagues are using synchrotron x-ray analysis at the Advanced Photon Source at Argonne to develop a three-dimensional image of cast iron alloys. He says, “We prepared a sample of a cast iron alloy that is two millimeters in diameter and 20 millimeters in height and then conducted a three-dimensional analysis of several segments. A series of radiographic images are taken at 0.2-degree intervals as the segment under evaluation undergoes a 180-degree rotation.”
The researchers are able to stitch all of these images together in a similar manner to a CT scan to provide a three-dimensional, volume analysis of the microstructure. The alloy studied is a compacted-graphite iron (CGI) that is used by one of Argonne’s industrial partners in the preparation of heavy-duty engine components.
The value of using x-rays is that they can clearly identify the components in the cast iron graphite microstructure. Singh says, “There is a clear absorption contrast between the graphite and iron in the microstructure. Graphite absorbs less x-ray energy that translates into a darker image as compared to iron, which is brighter.”
Figure 3 shows a three-dimensional model of the graphite particles in the CGI alloy studied. This image shows that synchrotron x-ray analysis can determine the morphology and size distribution of the graphite particles.
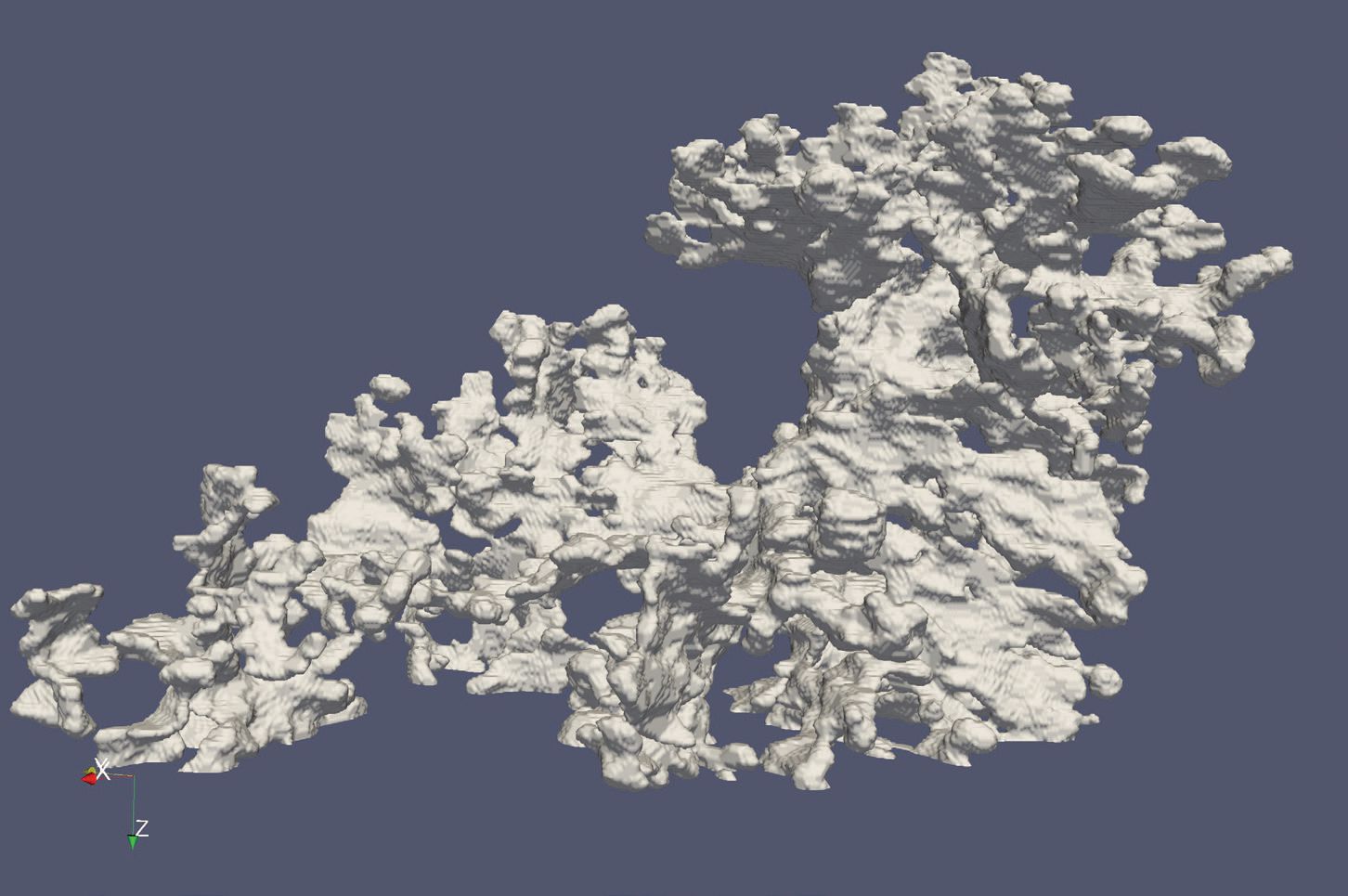 Figure 3. This three-dimensional image, produced by synchrotron x-ray analysis, promises to help researchers gain a better understanding of how structure influences the properties of cast iron. (Figure courtesy of Argonne National Laboratory.)
Figure 3. This three-dimensional image, produced by synchrotron x-ray analysis, promises to help researchers gain a better understanding of how structure influences the properties of cast iron. (Figure courtesy of Argonne National Laboratory.)
Singh says, “Two-dimensional analysis of cast iron does not provide a complete representation of the size and physical shape of the graphite particles present. Our approach can determine if a specific graphite particle is a nodule as opposed to being a CGI.”
For this particular alloy, the researchers found that the nodularity of the sample is 35% and the average diameter of nodular graphite is 12 microns. Singh is hopeful that synchrotron x-ray tomographic analysis can rapidly determine the real 3D structure of cast iron alloys, unlike current time consuming 2D sectioning techniques. He says, “We are hopeful that users of this technique will be able to use the structural information to develop the best possible cast iron alloy for their applications.”
Singh is interested in using synchrotron x-ray analysis to examine the structure of other alloys for structural applications in automotive and aerospace. Additional information can be found in a recent article (
2) or by contacting Singh at
dsingh@anl.gov.
REFERENCES
1.
Canter, N. (2015), “Vulnerability of titanium to oxygen,”
TLT, 71 (5), pp. 18-19.
2.
Chuang, C., Singh, D., Kenesei, P., Almer, J., Hryn, J. and Huff, R. (2015), “
Scripta Materialia,”
106, pp. 5-8.
 Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net.