Status of medium- and long-chain chlorinated paraffins
Dr. Neil Canter, Contributing Editor | TLT Feature Article March 2016
Will their use be restricted? And what are the alternatives?
KEY CONCEPTS
•
Uncertainty remains about how EPA will regulate chlorinated paraffins.
•
The MWF industry must accept that use of current chlorinated paraffins will probably end at some point in the future.
•
Combinations of alternatives are needed because no single alternative works in every case.
CHLORINATED PARAFFINS ARE THE MOST WIDELY USED EXTREME PRESSURE (EP) additive in the marketplace. They were introduced in the 1930s and are available in varying degrees of carbon chain length and chlorine content (
1).
Chlorinated paraffins are prepared from the reaction of chlorine with a paraffin or olefin of a specific chain length (
see Figure 1). The current types of chlorinated paraffins are distinguished by chain length ranges and are shown in Table 1.
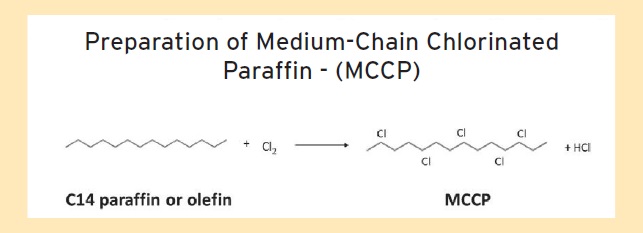 Figure 1. Chlorinated paraffins are produced through the reaction of chlorine with a paraffin or olefin as shown for an MCCP. (Figure courtesy of Qualice LLC.)
Figure 1. Chlorinated paraffins are produced through the reaction of chlorine with a paraffin or olefin as shown for an MCCP. (Figure courtesy of Qualice LLC.)
 Table 1. The current types of chlorinated paraffins are defined by specific chain length ranges. (Table courtesy of Chemical Solutions.)
Table 1. The current types of chlorinated paraffins are defined by specific chain length ranges. (Table courtesy of Chemical Solutions.)
Regulation of chlorinated paraffins started in the 1980s with the reporting of a U.S. National Toxicology Program study on a specific short-chain chlorinated paraffin (SCCP) and long-chain chlorinated paraffin (LCCP). A historical perspective on the regulation of chlorinated paraffins can be found in a recent STLE Webinar (
2) that was summarized in the February 2016 TLT.
In 2012 the U.S. Environmental Protection Agency (EPA) required, via a consent decree, that the chlorinated paraffin producers submit premanufacturing notifications (PMNs) for medium- and long-chain chlorinated paraffins (
3). As part of this arrangement, manufacturing and importation of SCCPs in the U.S. ceased. The metalworking fluid (MWF) industry had previously started to move away from this specific chlorinated paraffin chain in the mid-1990s when EPA ordered SCCPs to be subjected to Toxic Release Inventory reporting and mainly switched to medium-chain chlorinated paraffins (MCCPs) (
4).
The PMN process in the U.S. enables EPA to review “new substances” that companies want to introduce commercially and be listed on the TSCA Inventory. EPA indicated that in 2012 the chlorinated paraffin suppliers agreed to have medium- and long-chain chlorinated paraffins treated as new substances under TSCA section 5.
The big concern for EPA is that the currently used CAS numbers on the TSCA database for chlorinated paraffins are too general (
see Table 2). EPA believes that any chlorinated paraffins approved for use now and in the future must have CAS numbers with more specific chemical structure descriptions.
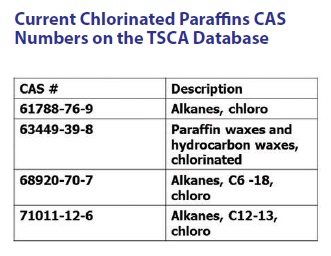 Table 2. The currently listed CAS numbers for chlorinated paraffins on the TSCA database are very general in description. (Table courtesy of Chemical Solutions.)
Table 2. The currently listed CAS numbers for chlorinated paraffins on the TSCA database are very general in description. (Table courtesy of Chemical Solutions.)
In 2012 and based on PMN submissions, EPA initiated a further risk assessment of MCCPs and LCCPs that was completed in November 2014. A redacted version of EPA’s risk assessment is now available through a link on the Independent Lubricant Manufacturer’s Association’s (ILMA) Website (
5). EPA’s preliminary conclusions are that MCCPs and LCCPs “present acute and chronic risks to aquatic organisms” and “may be very persistent and bioaccumulative.”
For these reasons, EPA in January 2015 gave chlorinated paraffin producers two options:
1.
Stop selling MCCPs and LCCPs immediately.
2.
Not produce or import MCCPs and LCCPs after May 31, 2016.
In effect, EPA will be impacting 99% of the chlorinated paraffins sold in the marketplace to the metalworking fluid industry. As part of a presentation from Dr. Maria Doa of EPA in September 2015, the agency has now decided to delay the stoppage of production and importation of MCCPs and LCCPs to mid-2017 (
6).
An important consideration is that no deadline has been established for when usage of MCCPs and LCCPs in the U.S. must cease. The metalworking fluid industry is waiting for EPA to develop a Significant New Use Rule (SNUR) to deal with the issue of critical applications for which acceptable substitutes are not available by the eventual ban date.
EPA is willing to obtain industry input on “critical uses” for MCCPs and LCCPs that might impact the agency’s final approach for dealing with these two additives. In December 2015, EPA published a notice in the Federal Register requesting new data for them to use in evaluation of the risk assessments for MCCPs and LCCPs (
7). Interested parties were asked to respond back with data and/or comments by Feb. 22, 2016.
A coalition of industry organizations has now been organized to respond to EPA’s action in (1.) withdrawing the general CAS numbers and descriptions for the chlorinated paraffins now on the TSCA Inventory and (2.) requiring producers and importers to submit PMNs so that they can be placed back on the TSCA Inventory under more specific CAS numbers and descriptions.
There is general chemical industry concern that EPA may use this approach on other chemical substances currently used commercially. The lead industry organizations working with EPA are the American Chemistry Council and ILMA. They are proposing three alternatives for EPA to consider in dealing with MCCPs and LCCPs.
1.
Complete the ongoing review of MCCPs and LCCPs under the TSCA work plan and if scientifically justified proceed with a TSCA section 6 rulemaking to impose disposal restrictions.
2.
After an opportunity for industry input, promote an SNUR imposing appropriate disposal restrictions.
3.
Obtain peer review of the draft risk assessment and issue a request for information on appropriate risk management controls for MCCPs and LCCPs.
As of the end of January 2016, the goal of the industry coalition remains to have EPA review MCCPs and LCCPs under TSCA section 6, which covers existing chemicals and enables the industry to provide EPA with input.
As of the end of January 2016, it is unclear exactly what will occur except that the MWF industry should realize that use of MCCPs and LCCPs will probably end at some point in the future.
This means that MWF formulators must reformulate their products to replace MCCPs and LCCPs with viable alternatives. As a follow-up to a past article on chlorinated paraffins (
8), the following companies were contacted again to provide updates on alternatives to chlorinated paraffins:
•
Afton Chemical Corp.
•
Croda Inc.
•
DIC Corp.
•
The Elco Corp.
•
The Lubrizol Corp.
•
Qualice LLC.
vLCCP
An example of EPA’s new approach on chlorinated paraffins is the recently commercialized very long-chain chlorinated paraffins (vLCCPs,
see Figure 2). As shown in Table 1, vLCCPs cover chlorinated paraffins with chain lengths between C21 and C30. STLE-member James MacNeil, product manager for Qualice LLC in Hamlet, N.C., says, “The paraffins used to prepare vLCCPs cannot contain more than 1% of a carbon chain length below C21.”
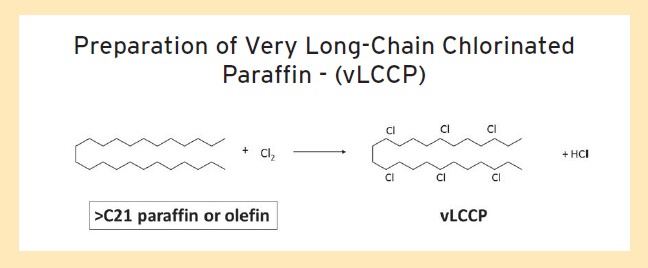 Figure 2. vLCCPs, currently cleared by EPA, are produced in the same manner as the other chlorinated paraffin types. (Figure courtesy of Qualice LLC.)
Figure 2. vLCCPs, currently cleared by EPA, are produced in the same manner as the other chlorinated paraffin types. (Figure courtesy of Qualice LLC.)
The vLCCPs currently cleared by EPA and commercialized are shown by CAS number and description in Table 3. These compounds are characterized by specific carbon chain lengths in contrast to the CAS numbers for the other chlorinated paraffins shown in Table 2.
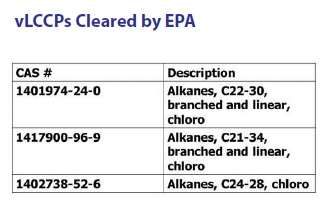 Table 3. The CAS numbers for vLCCPs listed on the TSCA database are defined by much more specific descriptions. (Table courtesy of Chemical Solutions.)
Table 3. The CAS numbers for vLCCPs listed on the TSCA database are defined by much more specific descriptions. (Table courtesy of Chemical Solutions.)
MacNeil says, “vLCCPs are fully registered under TSCA and are not under EPA risk assessment at this time.”
While comparable to MCCPs and LCCPs in chemical composition, vLCCPs exhibit higher molecular weights and as a consequence higher viscosities. MacNeil says, “vLCCPs have higher viscosities than mid-chain and long-chain chlorinated paraffins at equal chlorine content due to the length of the carbon chain. The phenomenon is familiar to compounders of metalworking fluids as a similar effect occurs in base oils.”
Formulating with vLCCPs can present some new challenges to the metalworking fluid formulator due to their higher chain length. MacNeil says, “vLCCPs have comparable solubility in oil for neat oil formulations as compared to MCCPs and LCCPs and also can be emulsified to create water-based metalworking fluids.”
An example of a sample emulsifiable oil formulation containing vLCCPs used at a treat rate of 10% is shown in Figure 3. MacNeil says, “We developed the formulation known as 94A with the additives shown using naphthenic oil as the base stock. The formulation is stable at a 5% dilution in 200 ppm and 400 ppm hard water as shown.”
 Figure 3. An emulsifiable oil concentrate prepared with vLCCP is shown on the left. The formulation is in the middle and 5% dilutions in 200 ppm and 400 ppm water are shown on the right. (Figure courtesy of Qualice LLC.)
Figure 3. An emulsifiable oil concentrate prepared with vLCCP is shown on the left. The formulation is in the middle and 5% dilutions in 200 ppm and 400 ppm water are shown on the right. (Figure courtesy of Qualice LLC.)
MacNeil considers vLCCPs to be a useful tool for the MWF formulator to consider as an alternative to LCCPs and MCCPs. He adds, “Some care in formulating will be needed with the higher viscosity of vLCCPs. Though the higher viscosity of vLCCPs dictates that their maximum chlorine content be around 50% by weight as opposed to 60% for an MCCP, the difference is quite small in a formulated product. At a treat rate of 20% in a formulated metalworking fluid, the 10% difference in chlorine content translates into a 2% difference if both chlorinated paraffins are used at a treat rate of 20% in the formulation.”
ALTERNATIVE CHLORINE-FREE EP ADDITIVES
There are a number of alternative chlorine-free EP additives that can be substituted for MCCPs and LCCPs in the main metalworking fluid types (neat oils, emulsifiable oils and semisynthetic fluids) where chlorinated paraffins are used. Most of the respondents list the following types as possibilities:
•
boundary lubricity additives (complex esters, polymeric esters and polyalkylene glycols)
•
phosphorous containing additives (phosphate esters, phosphites and phosphonates)
•
sulfur-based additives (sulfurized olefins, sulfurized fats, oils and esters)
•
overbased calcium sulfonates.
One of the challenges faced by metalworking fluid formulators is making sure that under a given set of operating conditions the chlorinated paraffin alternatives will exhibit comparable performance. In assessing boundary lubricity additives and extreme pressure additives, formulators must realize that they operate within different temperature ranges as shown in Figure 4.
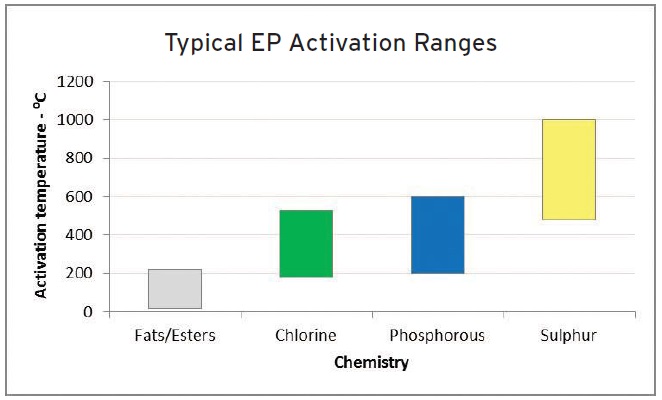 Figure 4. The different temperature ranges where boundary lubricity additives and extreme pressure additives operate are shown. (Figure courtesy of Afton Chemical.)
BENEFITS
Figure 4. The different temperature ranges where boundary lubricity additives and extreme pressure additives operate are shown. (Figure courtesy of Afton Chemical.)
BENEFITS
Anthony Jarvis, R&D manager, global MWF product development for Afton Chemical Corp. in Manchester, UK, indicates that alternatives to chlorinated paraffins offer advantages such as having regulatory approval for use, being able to be biodegradable, having greater ease of disposal, having a more favorable image from an environmental standpoint and having no chloride-based corrosion issues that chlorinated paraffins can face during application.
In the latter case, Jarvis says, “Chlorinated paraffin alternatives do not liberate hydrogen chloride during use, which reduces fumes and staining.”
But these alternatives also have some disadvantages. Jarvis says, “Alternatives for chlorinated paraffins can be less flexible in terms of applications and the choice of carrier base stocks (mineral oils and water) used in formulating them, have more difficulty in developing stable formulations, have less hard water stability and be more expensive.”
STLE-member Ben Faber, metalworking product manager for The Lubrizol Corp. in Wickliffe, Ohio, says, “Chlorinated paraffins undoubtedly provide great extreme pressure lubrication, but there are other alternatives that do not have the associated environmental and regulatory concerns.”
Faber believes that by switching to chlorine-free alternatives, formulators can achieve greater flexibility with their fluids. Extreme pressure additives can be combined to activate at broader temperatures that allows fluids to be used in more applications. In general, phosphorus additives activate at a lower temperature and sulfur additives activate at a higher temperature relative to chlorine.
“As part of this flexibility, formulators also may be able to use lower treat rates of additives such as sulfurized olefins and polymeric esters,” says STLE-member Gabe Kirsch, metalworking product manager for The Lubrizol Corp. He continues, “Some chlorine replacement chemistries are more compatible with use in water-based fluids, especially polymeric esters and phosphorus additives. Some additives require no emulsifier whatsoever and can allow for synthetics with improved lubricity.”
One other advantage for chlorinated paraffin alternatives cited by Kirsch is fewer staining and residue problems. He explains, “Through the machining process, chlorinated paraffin residues can cause undesirable ferrous corrosion, which is often overcome with non-chlorinated additives. In addition, chlorinated paraffin residues are notoriously difficult to remove in some applications, whereas chlorine-free alternatives are typically easier to clean off of a surface.”
STLE-member Larisa Marmerstein, R&D chemist for Elco Corp. in Cleveland, says, “Besides an improved environmental profile, chlorinated paraffin-free alternatives when used in metalworking fluids can contribute better tool life and finish on metal parts, easier cleaning and the ability to keep the cutting tool cooler especially in an emulsifiable oil.”
The last benefit is seen in a 4-ball EP ramp test (ASTM D2783) where the load is increased from 10 to 160 kgf over a period of 20 minutes. No heat is applied during the test.
Marmerstein says, “We evaluated an emulsifiable oil based on a polymeric phosphite ester versus an emulsifiable oil based on a chlorinated paraffin. The temperature changes over the 20-minute time frame. Figure 5 shows that the temperature of the chlorinated paraffin-free alternative fluid is more than 10 F lower than for the chlorinated paraffin-based fluid by the end of the test. The gap between the two fluids is as much as 40 F more than halfway through the test.”
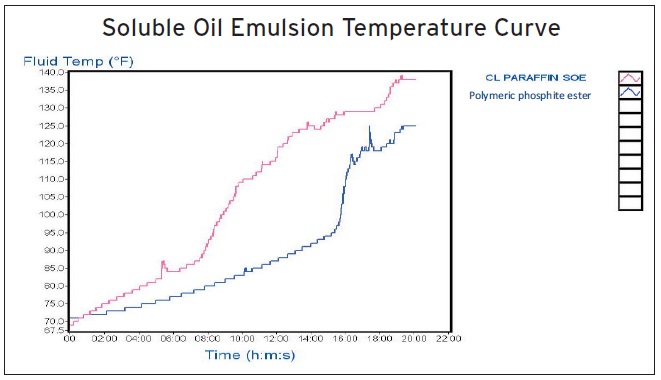 Figure 5. Results from a 4-ball EP ramp test (ASTM D2783) are shown comparing a chlorinated paraffin-based emulsifiable oil with a polymer phosphite ester-based emulsifiable oil. (Figure courtesy of Elco Corp.)
Figure 5. Results from a 4-ball EP ramp test (ASTM D2783) are shown comparing a chlorinated paraffin-based emulsifiable oil with a polymer phosphite ester-based emulsifiable oil. (Figure courtesy of Elco Corp.)
Hiroshi Sakata, manager polymer technical group for DIC Corp. in Chiba, Japan, believes that active and inactive sulfurized additives can provide benefits for formulators as chlorinated paraffin alternatives. He says, “For metal cutting applications, an inactive 30% sulfurized olefin can be effective in drilling, milling and turning operations in both neat oils and water dilutable fluids. In such metal forming applications as cold forming, stamping and fine blanking, the inactive 30% sulfurized olefin also can be effective. If the metal forming applications are compatible, a low-temperature, active 30% sulfurized olefin also can be useful because it performs well at keeping friction low over a wide range of loading.”
REPLACEMENT STEPS
STLE-member Mandi McElwain, lead application scientist-lubricants for Croda Inc. in New Castle, Del., provides guidance on how MWF formulators can move forward with replacing MCCPs and LCCPs. She says, “Formulators can start by directly replacing the chlorinated paraffins they are using with one of the alternatives. The next step is to evaluate the performance of their chlorinated paraffin-free formulation with modern and meaningful test methods such as the Microtap and the Twist Compression Test.
Depending on the application, it may be useful to evaluate the performance with more than one metal. In our experience, when certain complex esters are combined with phosphate esters or sulfurized olefins, synergies are present. So the formulator could similarly evaluate formulations containing more than one EP alternative.”
For example, Figure 6 shows Microtap data from the evaluation of cutting oils containing various additives on 1018 steel using forming taps. The treat rate for the various additives is shown on the left. McElwain says, “We found in this specific study that the combination of a complex ester with a phosphate ester displayed 14% lower torque at a lower treat rate than a cutting oil formulated with chlorinated paraffin.”
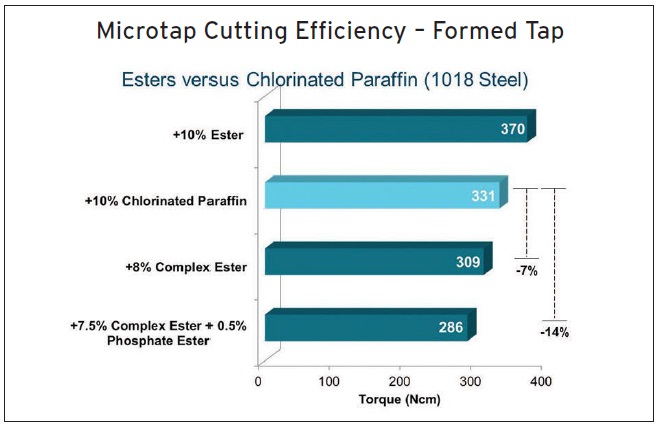 Figure 6. Microtap test data comparing straight oils formulated with different additives is shown. (Figure courtesy of Croda Inc.)
Figure 6. Microtap test data comparing straight oils formulated with different additives is shown. (Figure courtesy of Croda Inc.)
Sakata focuses on replacing chlorinated paraffins with sulfurized additives. He says, “The most important difference between sulfurized EP additives and chlorinated paraffins is the operating temperature of these additives. Since chlorinated paraffins react with metal surfaces at lower temperatures than sulfurized additives (
see Figure 4), chlorinated paraffins start to work at lower temperatures and loadings. Therefore when formulators work with sulfurized EP additives, they need to add additional additives to develop chlorinated paraffin-free fluids that exhibit comparable performance.”
Sakata continues by recommending specific additives that can work synergistically with sulfurized additives. He says, “For neat oils, overbased calcium sulfonate is an additive typically used with sulfurized additives. In forming fluids, several kinds of additives such as sulfurized fatty additives, non-sulfurized fatty additives, overbased calcium sulfonates, phosphate esters, etc., are added. It is very important for metalworking formulators to know what kind of synergistic effect can be obtained by mixing several additives to replace chlorinated paraffin.”
Figure 7 contains a tapping torque study done comparing the performance of 30% and 40% active sulfurized additives at a treat rate of 2.5% with 50% chlorinated paraffin at a comparable treat rate. Sakata says, “We evaluated the cutting oils on carbon steel and an aluminum alloy used in die casting. With both metal alloys, the 30%, low-temperature active sulfurized additive displays superior performance compared to the 50% chlorinated paraffin and the 40% sulfurized additive.”
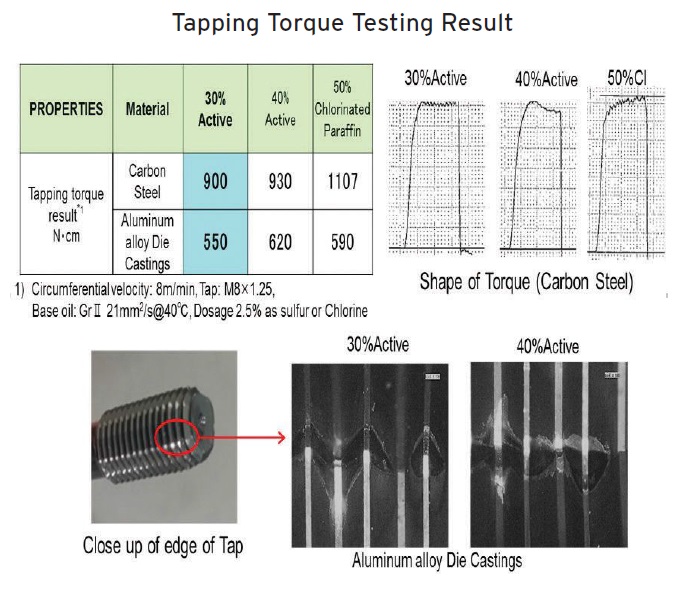 Figure 7. Three cutting oils prepared with different EP additives in Group II base oil are evaluated on carbon steel and an aluminum alloy in a tapping torque test. (Figure courtesy of DIC.)
Figure 7. Three cutting oils prepared with different EP additives in Group II base oil are evaluated on carbon steel and an aluminum alloy in a tapping torque test. (Figure courtesy of DIC.)
Faber offers five steps that a formulator should take to replace chlorinated paraffins in a specific application. He says, “The first step is for the formulator to find a lubrication test or tests that replicate the intended application. Then the next step is to identify the most effective chlorine replacement alternatives, and do not forget synergistic combinations of additives. In the case of water-based fluids, make sure that the emulsifier performance is balanced and the formulation makes a stable emulsion (adjust if necessary). The fourth step is to use laboratory bench tests to discriminate performance, and lastly lab tests should be confirmed with either in-house computerized numerical control machine testing or field trial testing that matches the intended application.”
Jarvis provides some guidelines for assisting formulators with replacing chlorinated paraffins. He says, “Generally if a system containing ≤10% chlorinated paraffins is being replaced, then for metal removal and forming processes this can be achieved with a reasonable degree of confidence. However, higher chlorinated paraffin-containing formulations become more difficult to predict.”
Jarvis continues, “Replacing chlorinated paraffins will probably require the formulator to completely re-examine all aspects of the formulation. It will require a complete matrix including various levels of EP and film strength additives.”
DIFFERENTIATE THE PERFORMANCE OF CHLORINE-FREE EP ADDITIVES
Marmerstein recommends that the formulator evaluate chlorinated paraffin-free formulations using a wide variety of tests. She says, “In addition to standard tests, we often use a modified 4-ball test, where the load on the ball is slowly increasing over a 20-minute period. This test is more severe than the regular 4-ball wear test and allows for better differentiation of additive effects in the fluids (
see Figure 5).”
Jarvis believes that lab-based tests should be performed on rudimentary oil/water emulsions to assess the inherent performance of the alternative additive compared to the chlorinated paraffin it is replacing. He adds, “A wide range of laboratory tests are available to evaluate the lubricity and EP characteristics of a fluid. Some examples include the Draw-Bead, Reichert, High Frequency Reciprocating Rig and the Hille Press.”
Figures 8 and 9 demonstrate the capabilities of the Hille Press test. Jarvis says, “This test involves the use of a laboratory scale deep drawing machine in which test blanks are coated with a lubricant, clamped at a fixed pressure and extruded through the use of a traveling punch. As shown in Figure 8, punch pressure (or resistance to deformation) is measured versus punch displacement until failure is reached. The objective is to find the lowest additive treat rate that displays the best combination of lowest punch pressure and highest punch displacement.”
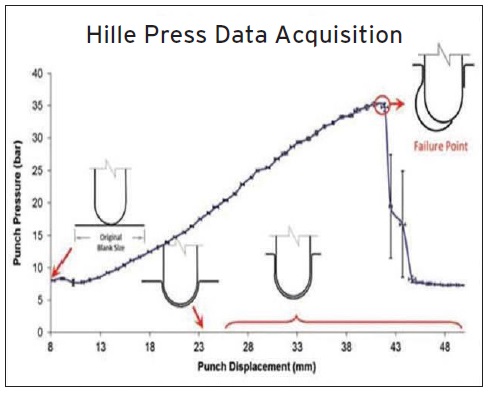 Figure 8. In the Hille Press test, punch pressure (or resistance to deformation) is measured versus punch displacement until failure is reached. (Figure courtesy of Afton Chemical.)
Figure 8. In the Hille Press test, punch pressure (or resistance to deformation) is measured versus punch displacement until failure is reached. (Figure courtesy of Afton Chemical.)
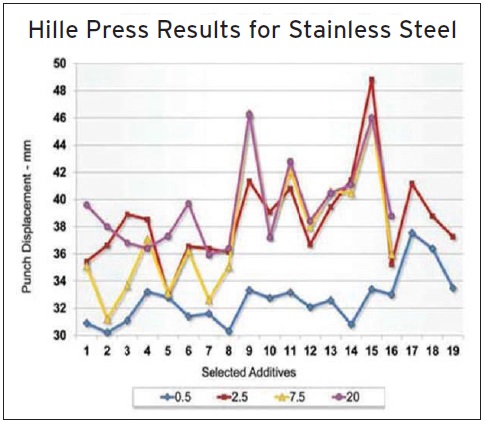 Figure 9. Hille Press test results on stainless steel for 19 additives evaluated at four treat rates is shown. (Figure courtesy of Afton Chemical.)
Figure 9. Hille Press test results on stainless steel for 19 additives evaluated at four treat rates is shown. (Figure courtesy of Afton Chemical.)
Typically an additive candidate is evaluated at treat rates of 0.5%, 2.5%, 7.5% and 20% in base oil and applied to the stainless steel blank (
see Figure 9). Jarvis says, “We use MCCP (additive 1) and two polymeric lubricant additives (additives 2 and 3) as industry standards. The remaining 16 additives are taken from a range of MWFs and other lubricants. High-performing additives display superior boundary lubrication and interact very effectively with metal surfaces.”
Kirsch says, “In addition to the tests previously described, we are investigating the use of traditional tribological tests including the mini traction machine and Cameron Plint to determine how best to evaluate alternatives to chlorinated paraffins.”
McElwain indicates that formulators need to evaluate other properties besides boundary lubricity and extreme pressure characteristics to determine if chlorinated paraffin-free alternatives will provide comparable performance. She says, “Formulators should evaluate such properties as concentrate stability, foam, tramp oil rejection and emulsion stability. If results from these bench tests and from lubricity tests are promising, then field performance will test the real-life viability of these chlorinated paraffin-free alternatives.”
EVALUATING SYNERGISTIC COMBINATIONS
Kirsch maintains that the MWF fluid industry has speculated for many years on the exact mechanism behind the synergies seen with various additive combinations. He says, “Extreme pressure additive performance can often be enhanced through synergy with other similar additives. By using materials that broadly function between 200-1,000 C, many to most metalworking and metal forming operations will gain improved tool life and processing speeds.”
Various synergisms are evaluated in a tapping torque test conducted on 1018 carbon steel. Five additive combinations are tested with LCCP being the reference. As shown in Figure 10, a combination of sulfurized olefin, overbased sulfonate and sulfurized fat displays the best performance.
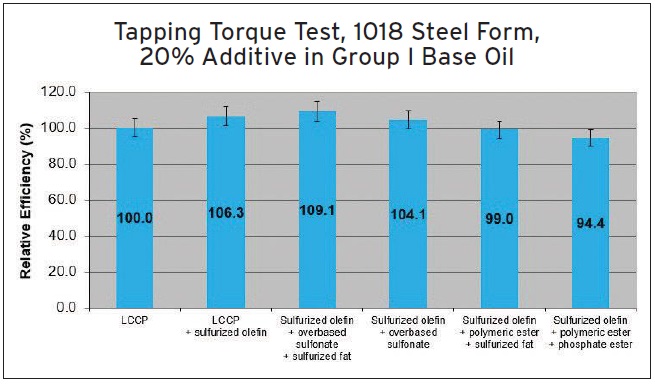 Figure 10. Five additive combinations blended in Group I base oil are evaluated with LCCP as the reference on 1018 carbon steel in a tapping torque test. (Figure courtesy of The Lubrizol Corp.)
Figure 10. Five additive combinations blended in Group I base oil are evaluated with LCCP as the reference on 1018 carbon steel in a tapping torque test. (Figure courtesy of The Lubrizol Corp.)
McElwain says, “We believe that certain additives have a higher propensity to bond ionically to the metal surface. This relative affinity may be impacted by temperature, explaining why EP additives ‘activate’ at different temperatures. When additives are used in combination, each additive may perform independent of the other or synergistically.”
Marmerstein says, “Phosphite esters work synergistically with sulfurized olefins and triglycerides, showing significant improvement in extreme pressure and tapping torque results.”
Jarvis cautions that further research must be done to clearly identify additive synergisms. He says, “More work is required on the mechanisms by which certain chlorinated paraffin replacement technologies work synergistically when in combination with each other.”
FORMULATOR PERSPECTIVE
With many options to choose to replace chlorinated paraffins, metalworking fluid formulators are faced with a major challenge in replacing MCCPs and LCCPs in their products. Former STLE President Jerry Byers, now an independent consultant in The Villages, Fla., says, “Formulators that typically have at least 200 products are faced with having to deal with formulations that can contain between 15-20 ingredients.”
Regulations are restricting the number of ingredients available to the formulator, which is making the challenge harder. “In effect, it is like trying to write a novel with fewer and fewer letters in the alphabet,” says Byers, editor of Metalworking Fluids, Second Edition (available at
www.stle.org). See Figure 11.
 Figure 11. The analogy can be drawn that the fewer ingredients available to formulators due to regulations is comparable to writing a novel without a complete alphabet. (Figure courtesy of Jerry Byers.)
Figure 11. The analogy can be drawn that the fewer ingredients available to formulators due to regulations is comparable to writing a novel without a complete alphabet. (Figure courtesy of Jerry Byers.)
MCCPs and LCCPs exhibit a great degree of flexibility that enables them to be used in a wide range of fluids and applications. While replacing them with alternative EP additives is do-able in most cases, Byers points out that there are applications that need chlorinated paraffins. He says, “Operations where chlorinated paraffins are needed include military applications (such as aviation fasteners), heavy-duty stamping and drawing, fine blanking, forming high strength, heavy gauge steels, older dyes, heavy-duty broaching and machining stainless steel. In the latter case, chlorinated paraffins are the only effective EP additive for this particular class of metal alloys.”
Alternative additives available to the formulator are listed in Table 4 by metalworking fluid type. While there appears to be a wide choice of options, each alternative additive exhibits some weaknesses.
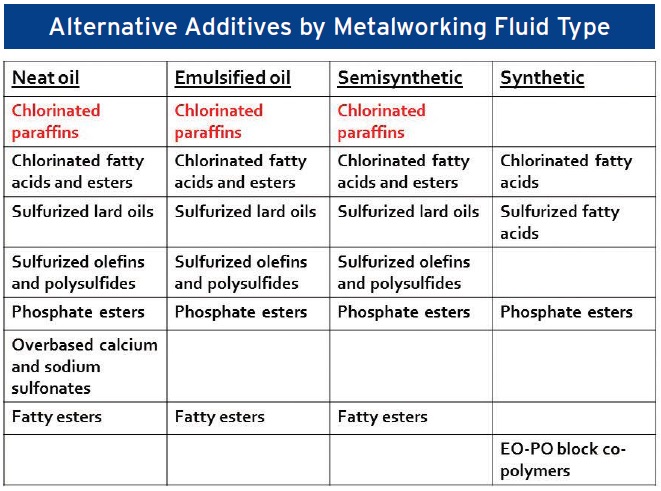 Table 4. Alternative additives available to the formulator are listed by metalworking fluid type. (Table courtesy of Jerry Byers.)
Table 4. Alternative additives available to the formulator are listed by metalworking fluid type. (Table courtesy of Jerry Byers.)
Byers says, “Sulfurized EP additives can generate odors that are objectionable and even dangerous since they can originate from the presence of hydrogen sulfide. This additive type also can stimulate microbial growth, cause ferrous corrosion, foam and stain copper alloys. Use of sulfurized additives with nickel-based alloys should be avoided because of the danger of forming a low melting point eutectic that weakens the metal surface.”
The main phosphorus-based EP additive is phosphate esters, which are known to stimulate microbial growth and generate foam in water-based metalworking fluids. Byers says, “Phosphate esters also can be too reactive with other components in the formulation and create problems when used in straight oils, including causing hydrolysis if water contamination is present, act to emulsify water and require high maintenance.”
Other alternative additives also have weaknesses including fatty esters that can readily hydrolyze in the alkaline environment of metalworking fluids, forming metal soaps that can lead to foaming and concentrate instability over time.
Byers sums up the challenges facing formulators: “Combinations of alternative additives will be needed because no single alternative will work in every case and each of the alternatives has problems. Product costs will increase by as much as 50%. But formulators should expect shorter sump life, higher disposal volumes and higher biocide usage.”
Additional details on the formulator’s perspective can be found in a recent presentation (
9).
FUTURE FOR ALTERNATIVE EP ADDITIVES
As the metalworking fluid industry turns to finding alternatives for MCCPs and LCCPs, the respondents were asked to predict what extreme pressure additives will be available for formulators in the future.
McElwain says, “Formulators will need to rely on existing chlorinated paraffin-free extreme pressure additives in the near future. New product introductions will take place in more of an evolutionary as opposed to revolutionary manner as the metalworking fluid industry gains a better handle on what applications will need better upgrades from MCCPs and LCCPs.”
Kirsch feels that finding additive synergies will be the key to replacing MCCPs and LCCPs. He says, “As machining and stamping operations increase in operational severity (e.g., higher pressures, faster speeds and feeds, etc.), fluids need to respond to this demand without compromising part and tooling integrity. Many extreme pressure additive alternatives to chlorinated paraffins are already available to the formulator. Synergies between these additives can often provide effective solutions for the formulator.”
Jarvis sees the future from the short/medium- and long-term perspectives. He says, “Over the short/medium-term, the current chlorinated paraffin-free EP additives will need to be used. Additive suppliers will be utilizing their R&D programs to develop newer approaches over the long term.”
Marmerstein predicts that formulators will be working with combinations of various types of chlorinated paraffin-free EP additives. She says, “Various sulfur, phosphorus and nitrogen components will be used in combination. For severe applications, formulators will work with active sulfurized olefins and fats in combination with phosphorus and amine chemistries to achieve improved lubricity, antiwear and lower friction.”
The current uncertainty about when MCCPs and LCCPs will be phased out is not stopping formulators from seeking and evaluating alternatives. This process will continue now and in the near future at a steady pace that may need to be accelerated depending upon when the use of main chlorinated paraffin types must cease in the U.S.
REFERENCES
1.
Kelley, T., Fensterheim, R. and Jaques, A. (2009), “Chlorinated paraffins,”
Compoundings,
59 (4), pp. 21-23.
2.
Canter, N. (2015), “Chlorinated paraffins: Where do we go from here?” STLE Webinar, June 10, 2015.
3.
Please click
here.
4.
In December 2014, EPA issued a notice requiring any future use of SCCPs required an SNUR.
5.
Please click
here.
6.
Doa, M. (Sept. 2015), “Chlorinated paraffins,” Presented at the 5th International Conference on Metal Removal Fluids in Chicago, Ill.
7.
EPA. (December 23, 2015), “Chlorinated paraffins: Request for available information on PMN risk assessments,”
Federal Register 80 (246), pp. 79886-79888.
8.
Canter, N. (2014), “EP additives: Regulatory updates of chlorinated paraffins and options on alternatives,” TLT,
70 (9), pp. 10-19.
9.
Byers, J. (Sept. 2015), “Formulator’s perspective: How to move on from chlorinated paraffins,” Presented at the 5th International Conference on Metal Removal Fluids in Chicago, Ill.
 Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. You can reach him at neilcanter@comcast.net
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. You can reach him at neilcanter@comcast.net.