Magnetocaloric effect using high-entropy alloys
Dr. Neil Canter, Contributing Editor | TLT Tech Beat February 2016
A high-entropy alloy exhibits the magnetocaloric effect at room temperature.
KEY CONCEPTS
•
A metal alloy exhibiting the magnetocaloric effect can act as a magnetic refrigerator that operates without a refrigerant.
•
Most of the currently available materials that are magnetocaloric are based on rare earth metals that have some negative characteristics.
•
A high-entropy alloy that contains no rare earth metals demonstrates the magnetocaloric effect at room temperature.
CONCERN WITH CONVENTIONAL REFRIGERATION PROCESSING, WHICH UTILIZES REFRIGERANTS found to have negative impacts on the environment, is leading researchers to evaluate alternative approaches. One area that is showing promise is to identify metal alloys that can absorb and then release heat when interacting with a magnetic field.
This phenomenon is known as magnetocaloric effect. Casey Miller, director for the MS Program in Materials Science and Engineering and associate professor in the School of Chemistry and Materials Science at Rochester Institute of Technology in Rochester, N.Y., says, “The magnetocaloric effect allows magnetic fields to alter the temperature of a material; this is greatest just above the magnetic ordering temperature of a ferromagnet. At such temperatures, an applied magnetic field will cause the magnetic moments to become less random. This decrease in magnetic entropy must be compensated by an increase of the lattice entropy, which is manifested as a temperature increase of the magnet. Conversely, removing a magnetic field decreases the material’s temperature to make up for the increased magnetic entropy (
see Figure 3). Cycling the field and taking appropriate care of the heat flow can thus form a magnetic refrigerator, prototypes of which have demonstrated efficiencies in excess of 60% of the Carnot limit—more than double conventional techniques.”
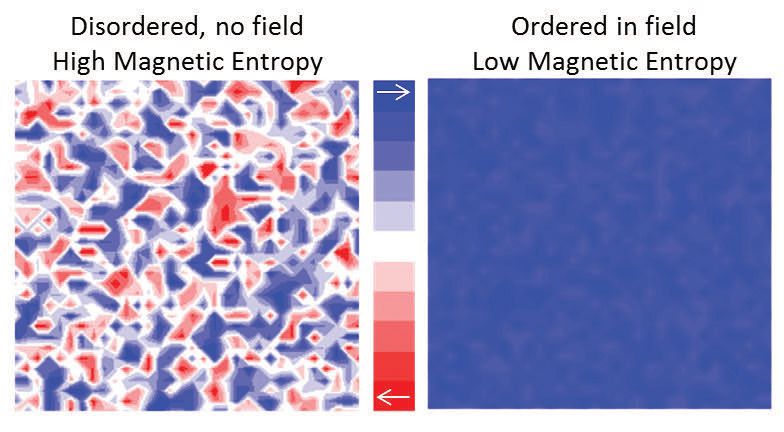 Figure 3. The local magnetic moment is disordered in no field (on the left), but becomes ordered in an applied magnetic field (on the right). This transition can be accompanied by a temperature change in magnetocaloric materials. (Figure courtesy of the Rochester Institute of Technology.)
Figure 3. The local magnetic moment is disordered in no field (on the left), but becomes ordered in an applied magnetic field (on the right). This transition can be accompanied by a temperature change in magnetocaloric materials. (Figure courtesy of the Rochester Institute of Technology.)
Most of the currently studied metal alloys that are magnetocaloric are based on rare earth metals. Miller says, “Rare earth metals are attractive for the magnetocaloric effect because the group contains some of the highest magnetic moments per atom on the periodic table. The problem with using them is that they are relatively expensive, subject to geopolitical pressures, oxidize easily and are very susceptible to corrosion.”
Miller also points out that rare earth metals have mechanical issues such as lack of ductility, which makes them difficult to use. He says, “One related concern is that this metal type also is difficult to machine.”
An example of a rare earth metal used as a magnetocaloric is gadolinium. Miller says, “This metal has an additional problem in that it generates sparks when sand blasted.”
In a previous TLT article, researchers facing this situation discovered a metal alloy based on manganese, iron, phosphorus and germanium that displays the magnetocaloric effect (
1). The reason this alloy is attractive is because it displays the magnetocaloric effect at room temperature and is less expensive than any alloy prepared from a rare earth metal.
Recently a new type of alloy known as high-entropy alloy was discovered. Miller says, “We know that these alloys have not been examined for the magnetocaloric effect and felt that it was worth evaluating them because there are a wide variety of types that can be tested. Our objective is to find a high-entropy alloy that will exhibit the magnetocaloric effect around room temperature.”
Miller feels that high-entropy alloys have far superior mechanical properties compared to rare earth metals and are easier to machine. Research documented in another previous TLT article supports Miller’s opinion about high-entropy alloys (
2). An alloy containing equal percentages of aluminum, lithium, magnesium, scandium and titanium exhibits a higher strength-to-weight ratio than any other existing metal.
A high-entropy alloy has now been identified that exhibits the magnetocaloric effect at room temperature.
TUNABILITY
Miller and his colleagues have determined that a high-entropy alloy prepared with equal parts of iron, cobalt, nickel and chromium in combination with palladium exhibits the magnetocaloric effect at room temperature. He says, “Palladium has the ability to tune the Curie temperature or critical temperature of the metal alloy, which is the temperature at which a material loses its permanent magnetic properties. By changing the concentration of palladium in the alloy, we can tune the critical temperature giving us the ability to dictate the temperature at which high-entropy alloys engage in the magnetocaloric effect.”
Palladium accomplishes this role by changing the crystal lattice. Miller says, “Small changes in the crystal structure generate large differences in magnetic properties.”
The high-entropy alloy is prepared by mixing the five elements in an arc furnace under an argon atmosphere. After cold rolling, which was done to process the alloy into thin sheets, the alloy is sealed in a quartz tube with argon gas and annealed at 900 C for one hour.
Miller says, “The rolled alloy is full of crystal defects. Annealing removes these dislocations, which do tend to affect the magnetic properties of the alloy.”
The researchers measured the magnetic phase transition as a function of temperature for the high-entropy alloys prepared with palladium concentrations ranging from 0-0.5 equivalent. Miller says, “Our objective is to maximize the magnetic entropy change.”
The researchers found that the alloy prepared with 0.5 equivalent of palladium yields the maximum entropy change, which is still about a factor of 20 less than that seen with gadolinium. Future work will focus on evaluation of other face-center cubic metals besides palladium to determine how they can be used to tune the magnetic properties of the alloy.
Miller says, “We hypothesize that substituting other face-centered cubic metals such as aluminum, copper and gold may lead to even stronger magnetocaloric effects while further reducing cost.”
Additional details on the research done with the high-entropy alloy can be found in a recent reference (
3) or by contacting Miller at
cwmsch@rit.edu. A second paper that was recently published discusses theoretical work done to predict the magnetocaloric effect for high-entropy alloys prepared with face-centered cubic transition metal alloys (
4).
REFERENCES
1.
Canter, N. (2009), “Magnetic refrigeration: Another way to cool,” TLT,
65 (5), pp. 12-13.
2.
Canter, N. (2015), “High-entropy alloys,” TLT,
71 (3), pp. 14-15.
3.
Belyea, D., Lucas. M., Michel, E., Horwath, J. and Miller, C. (2015), “Tunable magnetocaloric effect in transition metal alloys,”
Scientific Reports, 5:15755, DOI: 10.1038/srep15755.
4.
Kormann, F., Ma, D., Belyea, D., Lucas, M., Miller, C., Grabowski, B. and Sluiter, M. (2015), “‘Treasure maps’ for magnetic high-entropy alloys from theory and experiment,”
Applied Physics Letters,
107 (14), 142404.
 Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net.