Want to mix oil and water? It’s easy—sort of
Dr. Robert M. Gresham, Contributing Editor | TLT Lubrication Fundamentals January 2016
Surface active agents allow us to mix incompatible materials in a generally synergistic manner.
ACCORDING TO CONVENTIONAL WISDOM, "YOU CAN'T MIX OIL AND WATER.” And while in truth most oils and water have some very minute solubility in each other—certainly on a macroscale—you really can’t mix the two to get a true solution. Both Mother Nature and mankind, trading on the old DuPont adage, “Better Living Through Chemistry,” have learned to come pretty close. What I’m referring to, of course, is the wonderful and fascinating world of surface chemistry.
As tribologists we are concerned very much with certain aspects of surface chemistry: wear processes, tribochemical reactions, friction, atomistic processes, etc.—the whole nano world where certain atoms or molecules on surfaces come in contact with other chemicals to produce some pretty interesting results. For the oil and water case, the surface chemistry I’m referring to is the chemistry of surfactants—surface active agents. Surface active agents allow us to mix incompatible materials in a generally synergistic manner for some purpose.
There are all kinds of materials around us that derive some or all of their properties from the use of surfactants: engine oils, metalworking fluids, certain other lubricants, a wide variety of face creams, lotions, conditioners and cosmetics, shaving cream, water-based paints and coatings, a wide variety of food products conjured by man and, in fact, almost anything that is a water-based nonsolution. We all know chemical companies do some pretty amazing things, but what about Mother Nature? One of the first examples that we all encounter virtually at birth is the most obvious, common and best formulated: mammalian milk.
To understand all this, we need some definitions. Yes, I know there is a bit to digest here:
Wetting. The spreading and penetrating properties of a liquid by lowering its surface tension—that is, the tendency of its molecules to adhere to each other (see
surface tension for examples of wetting).
A suspension. The condition of a substance whose particles are dispersed through a fluid but not dissolved in it; the condition of having relatively large particles that will separate out on standing. These are materials that always say “shake well before using” on the labels. In nature, a muddy river might be an example—once it stops flowing, the mud settles and the water is clear again.
A dispersion. A near-colloidal system with its dispersed particles and the medium in which these are suspended. These are materials that sort of fit in the middle between colloids and suspensions. Most dispersions need or should be shaken or stirred before use. Some relatively stable salad dressings come immediately to mind.
A colloid. A solid, liquid or gaseous substance made up of very small, insoluble particles (as single large molecules or masses of smaller molecules) that remain in suspension in a surrounding solid, liquid or gaseous medium of different matter. Colloids usually are pretty stable; thus, a stable colloidal suspension such as homogenized milk, consisting of an immiscible liquid/solid (butter fat) dispersed and held in another liquid by substances called emulsifiers. Emulsions are a colloidal suspension of an insoluble liquid in another liquid.
Surface tension. The effect within the surface layer of a liquid that causes that layer to behave as an elastic sheet. It allows insects to walk on water. It allows small metal objects such as needles, razor blades or foil fragments to float on water, and it is the cause of capillary action. Whenever a rain drop falls forming a spherical droplet, a cleaning agent is mixed with water or an alcoholic beverage is stirred in a glass, the effects of surface tension are readily visible. Everyone is familiar with water droplets forming large beads (because of water’s high surface tension ~72) on a waxed surface (low surface tension ~<22), like a clean, freshly waxed automobile hood. The opposite phenomena is wetting where two surfaces of similar surface tension come together. The physical and chemical behavior of liquids cannot be understood without taking surface tension into account. It governs the shape that small masses of liquid can assume and
the degree of contact a liquid can make with another substance.
A surfactant. A substance, such as an emulsifier, detergent or wetting agent, that lowers the surface tension of the solvent in which it is dissolved or
the tension at the interface between two immiscible liquids. Add just a little soap to the water in the example above and the water no longer beads on the car hood but forms a smooth sheet.
Okay, so let’s try to cut to the chase. If we want to mix oil and water, we can do a poor job by making a suspension—depending on the nature of the oil maybe by just shaking it up a lot and it will be stable for a little while. Or we can make a dispersion by adding a
surfactant and stirring or shaking (mixing with a fair amount of energy). Or, we can make an emulsion with a different surfactant (a better one for our particular system—an emulsifier), mixing with some energy, resulting in a stable emulsion—and Abracadabra! We have mixed oil and water (
see Figure 1).
 Figure 1. We can make an emulsion with a different surfactant, mixing with some energy, resulting in a stable emulsion—and oil and water will be mixed. (Alchemist by Nicole Cardiff. Taken from http://fantasygallery.net/cardiff/art_12_alchemist-by-Nicole-Cardiff.html.)
Figure 1. We can make an emulsion with a different surfactant, mixing with some energy, resulting in a stable emulsion—and oil and water will be mixed. (Alchemist by Nicole Cardiff. Taken from http://fantasygallery.net/cardiff/art_12_alchemist-by-Nicole-Cardiff.html.)
Now let’s strip away the alchemist’s robes, cowl, incantations and crow feather and look at what’s going on. First, the surfactant is a molecule that typically has a long carbon chain tail with some kind of polar head attached (
see Figure 2). It’s kind of like a piece of spaghetti with something very different from pasta on the end of it.
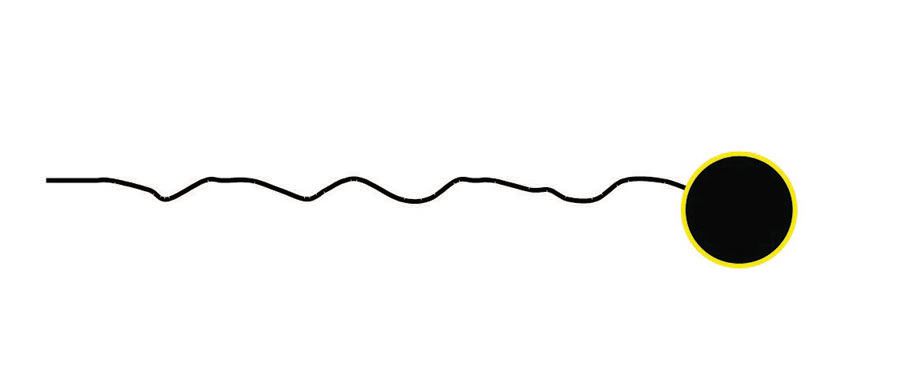 Figure 2. The surfactant is a molecule that typically has a long carbon chain tail with some kind of polar head.
Figure 2. The surfactant is a molecule that typically has a long carbon chain tail with some kind of polar head.
The polar head generally likes polar solvents like water and the hydrocarbon tail generally likes nonpolar solvents like oil or other similar long straight chain nonpolar chemicals. Polar solvents and functional groups on molecules generally have some kind of ionic character: plus or minus charges, partial plus or minus charges or just atoms with a lot of loosely held electrons. The polar head of the surfactant likes water and so aligns itself like a “ball” in the water with the polar heads on the outside (because they like water) and the long nonpolar tails to the inside (because they don’t like water). These structures are called micelles. In nonpolar solvents, they do just the reverse (that is, the polar heads align to the interior of the “ball” and the nonpolar tail to the outside). Okay, so what does this have to do with mixing oil and water? Well, in a polar solvent, the oil would be happiest in the center of the micelle because the long straight carbon chains are like the oil molecules. Conversely, if we had only a little water in our oil, the micelle would reverse and the water would move to the interior of the micelle. When this happens to the micelle in a chemical system we call it an inversion. While these descriptions are a little simplistic, the idea is that whether we have oil, dirt, gasses, chemicals, etc., a polar material is made more or less compatible with a nonpolar material. Included are some visual examples that might make this easier to understand.
Imagine motor oil with a dispersant, as most have. The engine dirt tends to be polar, so the polar heads of the dispersant attach to the polar parts of the dirt, helping to keep it in suspension in the oil until it’s time for an oil change or the particles agglomerate to a large enough size to catch in the oil filter (
see Figure 3).
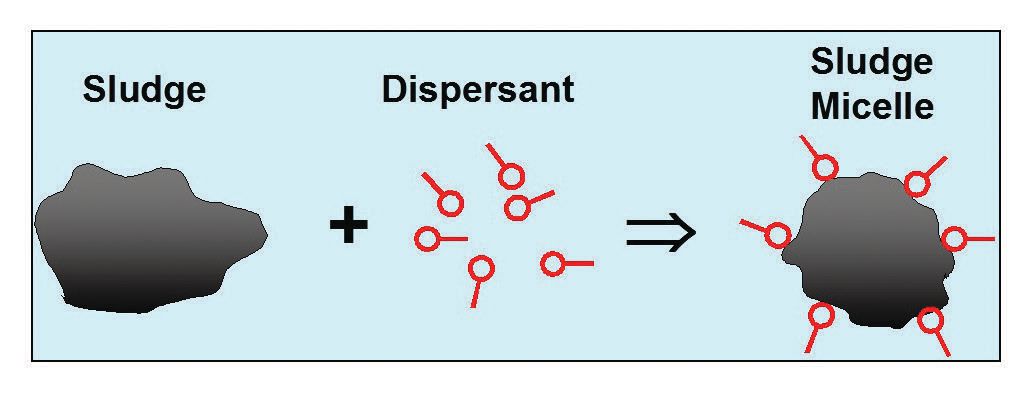 Figure 3. The engine dirt tends to be polar, so the polar heads of the dispersant attach to the polar parts of the dirt, helping to keep it in suspension in the oil.
Figure 3. The engine dirt tends to be polar, so the polar heads of the dispersant attach to the polar parts of the dirt, helping to keep it in suspension in the oil.
Or, as shown in Figure 4, imagine very polar calcium carbonate (CaCO
3 with positive charges on calcium) dispersed in a nonpolar solvent, like oil, hexane or the like, and containing a dispersant that has a polar sulfonate head (SO
3- with a minus charge). Thus, the nonpolar tails are to the outside of the micelle making the calcium carbonate compatible with the nonpolar solvent. Hopefully a pattern begins to emerge.
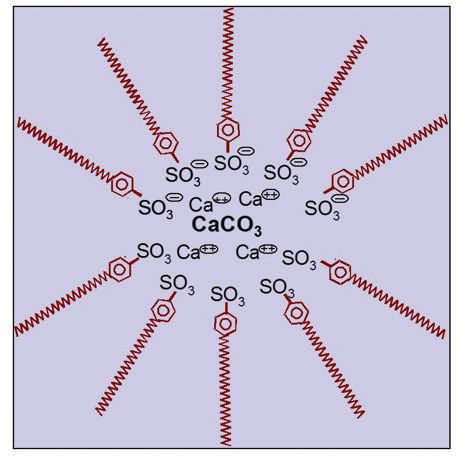 Figure 4. Very polar calcium carbonate dispersed in a nonpolar solvent, like oil, hexane or the like, and containing a dispersant that has a polar sulfonate head.
Figure 4. Very polar calcium carbonate dispersed in a nonpolar solvent, like oil, hexane or the like, and containing a dispersant that has a polar sulfonate head.
One of the key things that characterizes the difference between suspensions, dispersions and emulsions is their inherent stability or tendency not to separate or settle. Aside from the relative goodness of the surfactant, of prime importance is the particle size (whether gasses, solids or liquids) of the material being dispersed. They settle because the particle size is too large and/or not enough surfactant is on the surface to keep them from settling. In colloidal emulsions, the particle sizes tend to be quite small, usually well less than you can see, with dispersions being larger and suspensions very large indeed. To make an emulsion, sometimes you must have vigorous mixing or even milling or grinding to get the particle size small enough (because small particles have more surface area relative to their mass than larger particles) to get enough surfactant on the surface to do the job.
As a further example, back in those thrilling days of yesteryear, as a young chemist, I developed a process for synthesizing a particular chemical. The final step in the process resulted in the chemical precipitating (coming out of solution) into a nice crystalline slurry (a coarse suspension) form that could be filtered like beach sand from the reaction mass, dried and sold as a final product.
When I scaled it up in the plant to 5,000 gallons, low and behold, instead of a nice slurry I got something that looked more like a gigantic beer head containing my product. Alas, I couldn’t pump it to the filter as the kettle drain was on the bottom and the “beer head” would hang up in the kettle and plug the drain. “It didn’t happen like that in the lab!” Ever hear that one before? I was lower than a snake’s belly in a wagon rut. Oh, woe! What to do?
Enter the MPS (magic pinch of s...—stuff), in this case a product called Triton100X (I have to use the tradename because I have forgotten the chemistry). I don’t know if it is still on the market, but it was an excellent, versatile surfactant. Anyway, I added an ounce to the kettle and SHAZAAM! I got my slurry of beach sand and off to the filter we went! What happened? The solids entrained small air bubbles resulting from the more vigorous mixing in the bigger kettle and from the differences in surface tension between my product, air and the reaction mass. The solids did not wet out; rather they had tiny air bubbles coating each crystal causing them to float. Addition of the surfactant caused the surface tension to change at the surface of the solids, the foam broke immediately, the slurry returned and my job was secured until the next crisis.
To carry the previous story a little further, Triton 100X also could be used in higher concentrations as a dispersant or emulsifier or, in much higher concentrations, as I understand it, to make a very stable foam for a commercial shaving cream! A great MPS!
We have a similar example in one of our case histories in STLE’s Metalworking Fluids Management Certificate Education Course, where metal fines are floating in a metalworking central system with the lack of wetting being the problem. Addition of the appropriate wetting agent solved the problem for the metalworking plant as well.
So now you know how to mix oil and water! You also know how to identify the effects of surface tension. That was the prime purpose of this article, and you can stop reading here.
But if you want extra credit, read on. What do you do if you
don’t want to mix oil and water—that is, you don’t want a stable emulsion? Ask your mom—she knows everything! When she runs out of buttermilk and can’t get to the store, she takes regular whole milk, adds a little vinegar, stirs it up and presto! She has buttermilk. What she did was break Mother Nature’s emulsion by adding acid, which ties up the polar heads of Ma Nature’s surfactant and the butter drops out in little globs. Mom is so smart!
Many metalworking fluids also are emulsions. The better the emulsion, the longer the fluid lasts in the system and the happier the customer. But eventually it is time to finally send that fluid to the waste treatment system. What happens?
Well, a really good emulsion just might go through the waste treatment plant (depending on its design) and out into the waste stream. Not good! So the waste treatment manager treats the emulsion with acid, the emulsion breaks and then the waste treatment system can capture and concentrate the waste, leaving the clean waste water stream safe for the environment.
So you want to mix oil and water—no problem. You don’t want to mix oil and water—no problem. As DuPont says, “Better Living Through Chemistry!”
 Bob Gresham is STLE’s director of professional development. You can reach him at rgresham@stle.org
Bob Gresham is STLE’s director of professional development. You can reach him at rgresham@stle.org.