More effective emissions catalyst at low temperatures
Dr. Neil Canter, Contributing Editor | TLT Tech Beat December 2015
A new catalyst can facilitate carbon monoxide oxidation without hydrocarbon interference.
KEY CONCEPTS
•
The greater efficiency in the combustion process means that the currently used precious metal emission catalysts must operate at lower temperatures where they are not as effective particularly in oxidizing carbon monoxide.
•
A ternary oxide based on copper, cobalt and cerium oxides has been found to be effective at oxidizing carbon monoxide at temperatures below 200 C.
•
The ternary oxide catalyst oxidizes carbon monoxide without hydrocarbon interference in contrast to precious metal catalysts.
THE EMPHASIS NOW PLACED ON IMPROVING THE FUEL ECONOMY of internal combustion engines is leading to exhaust conditions that are more challenging to treat. In the preparation of automotive engine oils, a key concern is lubricant compatibility with the currently used three-way catalytic converter derived from the precious metals palladium, platinum and rhodium.
A previous TLT article describes the development of a new catalytic converter based on microstructured ceramic substrates (
1). The new catalytic converter demonstrates better effectiveness, more durability and a larger geometric surface area with larger channel diameters leading to reduced fuel consumption. These advantages are all done using a lower level of precious metals than are currently used.
The emissions catalyst faces a new challenge of having to work effectively at lower temperatures. Dr. Andrew Binder, post-doctoral researcher at Oak Ridge National Laboratory in Oak Ridge, Tenn., says, “Greater efficiency in the combustion process means that less heat is generated and the temperature of the emissions stream is lower. This means that future engines will be operating at lower temperatures, and emission catalysts also will be required to operate effectively at these lower temperatures. The goal established by the automotive industry is to achieve a 90% conversion of pollutants at 150 C.”
Currently internal combustion engines operate at higher temperatures. Dr. Todd Toops, Distinguished R&D scientist at Oak Ridge National Laboratory, says, “The Ford F-250 diesel engine has a temperature profile that stabilizes at approximately 200 C.”
One problem seen at low temperatures (below 200 C) with the current precious metal catalysts is that hydrocarbons interfere with oxidation of carbon monoxide. Binder says, “Specific binding sites on the surface of the precious metal catalysts are used to oxidize carbon monoxide and hydrocarbons. The problem is that hydrocarbons bind to the precious metals at a lower temperature than carbon monoxide and are oxidized at a higher temperature than carbon monoxide.”
In effect, there is a competition between carbon monoxide and hydrocarbons for the same catalytic site. Hydrocarbons tie up the sites not allowing carbon monoxide to be oxidized.
A second problem is that precious metal catalyst can sinter at the high temperatures sometimes present in internal combustion engines. Toops says, “Sintering leads to agglomeration of the precious metal catalyst reducing surface area and as a consequence the nanoscale effectiveness of the catalyst.”
Development of a new catalyst that can facilitate carbon monoxide oxidation without hydrocarbon interference is desired. Such a catalyst has now been developed.
TERNARY OXIDE
Binder and Toops, in collaboration with colleagues, have determined that a catalyst containing copper oxide, cobalt oxide and cerium oxide is effective at oxidizing carbon monoxide at temperatures below 200 C in the presence of hydrocarbon. Binder says, “Previous work we have done in working with metal oxide systems led us to evaluate this ternary oxide combination due to its high activity and thermal stability.”
Catalyst synthesis was conducted through a precipitation process followed by filtration, washing, drying in a vacuum oven and calcinations at 600 C. A custom fixed bed reactor was used to evaluate the ternary oxide catalyst at temperatures ranging from 60 C to 600 C. These experiments simulated conditions present in a lean-burning diesel engine exhaust.
The representative hydrocarbon used by the researchers is propylene. Binder says, “Propylene is a commonly used model hydrocarbon that is a good benchmark. This hydrocarbon also is involved in the low temperature emissions evaluation profile.”
Annular dark field scanning transmission electron microscopy is one of the techniques used to characterize the catalyst as shown in the upper left hand corner of Figure 2. The catalyst contains distinctive cobalt oxide and cerium oxide regions. No copper or copper oxide is found and energy dispersive x-ray spectroscopy shows that this species is uniformly dispersed throughout both oxide regions. The remaining images in Figure 2 are energy dispersive x-ray elemental mapping of copper, cerium and cobalt.
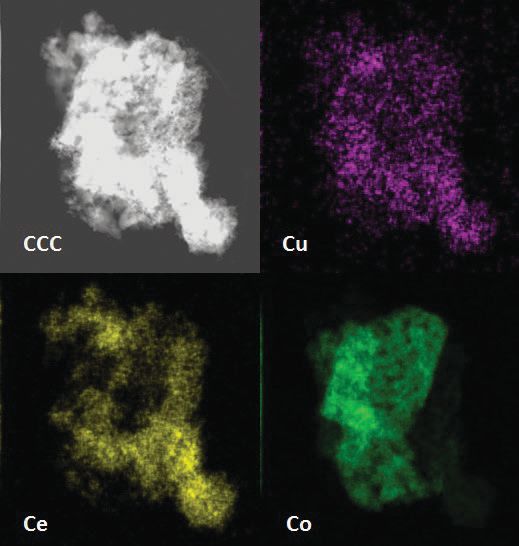 Figure 2. Annular dark field scanning transmission electron microscopy and energy dispersive x-ray elemental mapping of copper, cerium and cobalt are used to better understand the surface of the ternary oxide catalyst. (Figure courtesy of Oak Ridge National Laboratory.)
Figure 2. Annular dark field scanning transmission electron microscopy and energy dispersive x-ray elemental mapping of copper, cerium and cobalt are used to better understand the surface of the ternary oxide catalyst. (Figure courtesy of Oak Ridge National Laboratory.)
Toops says, “We anticipated that copper oxide would not form a distinctive region because the ternary oxide only contains 9% by weight of this species. We believe that the uniform dispersion of copper contributes to the performance of the catalyst because copper—if present in higher concentrations—can agglomerate.”
The researchers believe that there are two possible reasons that can explain how the ternary oxide performs. Binder says, “One reason for the good performance is that propylene does not adsorb as well to the ternary oxide as it does to precious metal catalysts. This means that carbon monoxide oxidation can take place unaffected by the presence of propylene.”
A second possibility is that the oxidation of carbon monoxide and of hydrocarbons occurs at two different sites on the surface of the ternary oxide. Binder says, “In results from testing of binary oxide combinations of the three metal oxide catalysts, copper-cerium and cobalt-cerium interface sites are the most important locations for carbon monoxide oxidation because both can provide oxygen through a weakened metal-oxygen bond. Propylene oxidation appears to take place primarily on the surface of cobalt oxide.”
Future research will be focused on determining the usability of the catalyst. Thermal aging and sulfur aging studies are already underway. Binder adds, “We also will be tweaking the ternary oxide formulation by changing the stoichiometry and adding another metal oxide.”
Further information can be found in a recent article (
2) or by contacting Binder at
binderaj@ornl.gov.
REFERENCES
1.
Canter, N. (2014), “New catalytic converter technology,” TLT,
70 (6), pp. 14-15.
2.
Binder, A., Toops, T., Unocic, R., Parks, J. and Dai, S. (2015), “Low temperature CO oxidation over ternary oxide with high resistance to hydrocarbon inhibition,”
Angewandte Chemie International Edition, DOI: 10.1002/anie.201506093.
 Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net.