Safer jet fuel
Dr. Neil Canter, Contributing Editor | TLT Tech Beat December 2015
A new additive could help reduce the risk of jet fuel mist fires.
KEY CONCEPTS
•
When in use, jet fuel is distributed into a fine mist that can easily be ignited.
•
A new additive known as a megasupramolecule has been developed that can control misting of jet fuel and reduce the risk of fire.
•
Unlike other multimillion molecular weight polymers, chains from the magasupramolecules can re-engage after shearing, enabling mist control to be retained and making the shearing process reversible.
WHEN MOST OF US FLY, WE ARE AWARE THAT HIGH-PERFORMANCE LUBRICANTS and fuel are required to meet the demanding requirements placed on an airplane. Jet fuel consists of a combination of hydrocarbons that include paraffins, cycloparaffins, naphthenes, aromatics and olefins.
In a recent TLT article, research was done on a specific type of jet fuel known as JP-8 that contains a higher level of sulfur and is used over a wide temperature range by the military (
1). The challenge faced by the researchers was to use a biofuel cell to convert JP-8 into useful energy at room temperature. Despite the potential interference of sulfur, which is known to poison metal catalysts, the researchers devised an enzyme cascade pathway to oxidize JP-8 into useful energy.
Julia Kornfield, professor of chemical engineering at the California Institute of Technology in Pasadena, Calif., says, “Jet fuel in its liquid state has a low-fire risk. But the fire danger increases when jet fuel is present as a fine mist. In engine operation, jet fuel is distributed into a mist that can easily be ignited, enabling the airplane to move. Problems arise when a mist is created outside the engine.”
Kornfield referred to the massive fire in the fuel storage system at Miami International Airport in March 2011. This incident was apparently caused by a failure of one of the large main jet fuel pumps when its manifold separated enough to spray jet fuel under extremely high pressure into the air, leading to the massive explosions that destroyed the fuel storage system and disrupted air travel.
While Jet-A fuel in civil aviation may contain an antistatic additive and military jet fuel is formulated with a number of additives at low concentration (pour point depressant, antioxidant, lubricity enhancer and metal deactivator), none of these additives can reduce the risk of fuel mist explosions and fires.
A new additive has been discovered that shows promise in reducing the risk of jet fuel mist fires.
MEGASUPRAMOLECULES
Kornfield and her colleagues have now developed a new type of additive that controls misting of jet fuel and reduces the risk of fire. She says, “The additive is based on a type of polymer that dynamically associates to form megasupramolecules that have a molecular weight in the millions of Daltons. The individual molecules contain about 50,000 background carbons and have end groups that can stick to one another.”
Kornfield indicated that this finding is in contrast to literature that limited end-to-end association to lower molecular weights. The long-end associative polymers are prepared by polymerization of cyclooctadiene using a method that places specific functionalities at both ends of each chain.
She says, “We were looking for ways to have end groups adhere to each other in a similar manner to Velcro to protect the polymers from degrading under high shearing conditions. End groups prepared with dicarboxylic acids and tertiary amines were found to fulfill this role by forming charge-assisted hydrogen bonds that are strong enough to drive formation of megasupramolecules and weak enough to release before tension gets high enough to break a covalent bond.”
In a similar manner to multimillion molecular weight polymers, megasupramolecules inhibit elongation and breakup of fuel droplets. The important difference is that the end groups can release when fuel passes through pumps and filters and re-engage as the chains meet again making the shearing process reversible and retaining the mist control properties of the polymer.
The megasupramolecules are characterized by methods such as light scattering and shear-flow viscometry to confirm their formation.
Evaluation of the megasupramolecules at a 0.3% concentration by weight in jet fuel was accomplished by using a 140 mile per hour projectile to disperse mist over continuously burning propane torches.
In the case of untreated jet fuel, a fireball is generated. With the addition of the megasupramolecule, no fireball is observed. The entire test is completed in approximately an eighth of a second. A video showing the testing can be found at the Website for Kornfield’s research group (
2).
Figure 1 shows the end of the test where the flame in the upper part of the image is generated for untreated jet fuel and the lack of a flame on the bottom occurs with jet fuel formulated with the megasupramolecule.
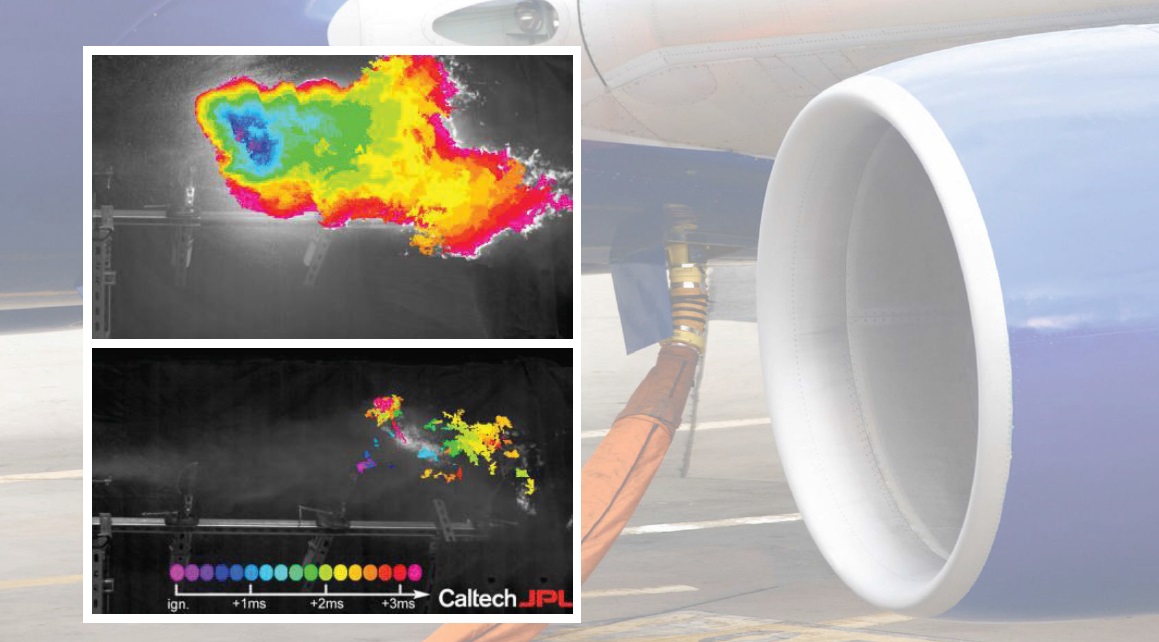 Figure 1. Incorporation of a new jet fuel additive known as a megasupramolecule reduces mist formation to the point where no flame is seen on the bottom after a 140 mile per hour projectile is used to cause jet fuel to disperse, while a fireball is observed on top with untreated jet fuel in the same experiment. (Figure courtesy of the California Institute of Technology.)
Figure 1. Incorporation of a new jet fuel additive known as a megasupramolecule reduces mist formation to the point where no flame is seen on the bottom after a 140 mile per hour projectile is used to cause jet fuel to disperse, while a fireball is observed on top with untreated jet fuel in the same experiment. (Figure courtesy of the California Institute of Technology.)
Kornfield is faced with two challenges in commercializing this meagsupramolecule additive. She says, “We have only produced enough polymer for tests in diesel engines, both a 2 kilowatt diesel generator and a 360 horsepower (270 kilowatt) diesel truck engine using a treat rate of 1000 pppm. We need to make a much larger quantity of this polymer in order to do an evaluation in a jet engine. The second challenge is to see if megasupramolecules can be effective in the parts per million treat rate where many other fuel additives are used.”
Kornfield points out that diesel engine testing showed that the megasupramolecule can reduce soot formation without affecting the performance of the fuel. She says, “We have also found that the megasupramolecule will reduce drag when fuel is moved through pipelines.”
This latter property suggests that the megasupramolecule may act as a lubricity additive. Additional information can be found in a recently published article (
3) or by contacting Kornfield at
jakornfield@cheme.caltech.edu.
REFERENCES
1.
Canter, N. (2015), “Room temperature biofuel cell,” TLT,
71 (2), pp. 14-15.
2.
Please see
https://kornfield.caltech.edu.
3.
Wei, M., Li, B., David, R., Jones, S., Sarohia, V., Schmitigal, J. and Kornfield, J. (2015), “Megasupramolecules for safer, cleaner fuel by end association of long telechelic polymers,”
Science,
350 (6256), pp. 72-75.
 Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net.