Effective nanocatalysts with reduced precious metal content
Dr. Neil Canter, Contributing Editor | TLT Tech Beat October 2015
A new type of nanocatalyst has been developed to maximize performance without reducing surface area.
KEY CONCEPTS
•
The efficiency of a catalyst is directly related to the percentage of atoms on the surface available to participate in a specific process such as the oxygen reduction reaction.
•
Application of a thin layer of platinum onto a palladium nanocrystal template followed by etching leads to the formation of hollow nanocage structures.
•
These hollow nanocages exhibit high-surface area, good catalytic activity and excellent durability.
PLATINUM IS ONE OF THE MOST WIDELY USED CATALYSTS FOR KEY PROCESSES such as automotive catalytic converters and fuel cells. But the high cost and low availability of platinum and other precious metals is leading researchers to seek ways to use less catalyst while maintaining activity.
Catalysts performance is measured by a parameter known as utilization efficiency (UE). Dr. Younan Xia, professor in the department of biomedical engineering, the School of Chemistry and Biochemistry and the School of Chemical and Biomolecular Engineering at Georgia Tech in Atlanta, says, “A typical metal particle for a catalyst contains about 100 atoms, but not all of them participate in catalysis because they are not all on the surface. UE indicates the percentage of atoms on the surface. All other atoms inside the particle are essentially wasted from a catalysis standpoint.”
Nanocrystals are attractive as catalyst candidates because they exhibit a higher surface area compared to macroscale catalysts. Xia says, “The one problem with nanocrystals is a high-surface energy that leads the particles to agglomerate in order to form a more thermodynamically stable state reducing the number of atoms available to act as catalysts.”
Other approaches have been taken to develop high-performing nanocatalysts. In a previous TLT article, researchers encapsulated gold clusters in a polyamidoamine-based dendrimer polymer that is placed on a mesoporous silica support to form a material that combines the benefits of heterogeneous and homogeneous catalysts (
1).
Xia indicates that prior research to develop more efficient nanocatalysts included the evaluation of nanoframes and nanosheets. He says, “Nanoframes refer to materials shaped like rods where multiple ridges of atoms as thin as a few nanometers are present. The problem is developing the proper surface geometry so that the catalyst displays the desired activity and selectivity. With nanosheets that are one or two atomic layers thick, stability is a problem that leads to the need for using bulky surfactants to stabilize them. Unfortunately, the bulky molecules needed to stabilize the nanosheets also reduce the catalyst’s surface area.”
A new type of nanocatalyst is needed to maximize performance without reducing surface area. Such a nanocatalyst has now been developed.
HOLLOW NANOCAGES
Xia and his research colleagues use a new technique to fabricate platinum nanocatalysts that exhibit high-surface area, good catalytic activity and strong durability. He says, “We applied a thin layer of platinum three layers thick on a palladium nanocrystal template and then removed the latter through an etching process to form hollow nanocage structures. The porous structure enables atoms both on the outside and inside of the nanocages to generate catalytic activity so the UE is very high.”
A platinum precursor is reduced and deposited as platinum atoms onto palladium cubes at 200 C. Under these conditions, cubic arrays of platinum atoms are formed on a flat surface. After etching removes the palladium templates, the platinum atoms in a 100 orientation form cubic nanocages. Figure 3 shows a transmission electron microscope image of the cubic nanocages, which are each about 20 nanometers in diameter.
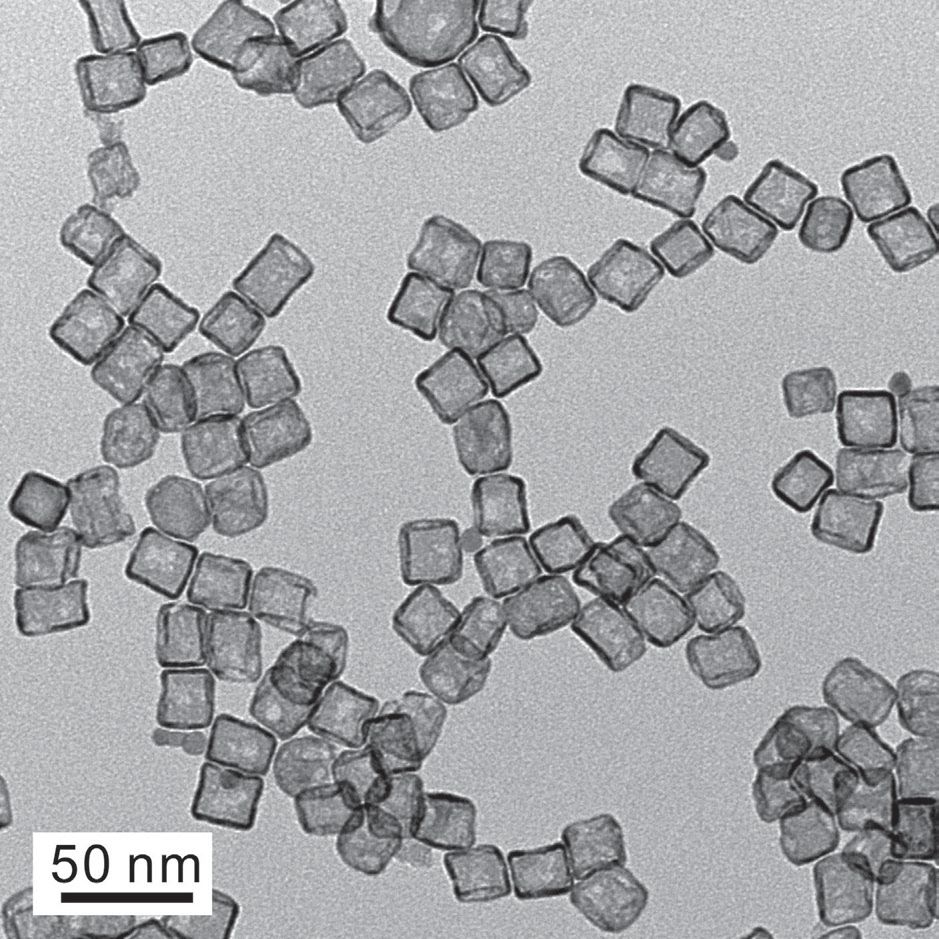 Figure 3. A transmission electron microscope image shows platinum hollow nanocages that exhibit electrocatalytic activity that is five times better than a standard platinum/carbon catalyst in an oxygen reduction reaction. (Figure courtesy of Georgia Tech.)
Figure 3. A transmission electron microscope image shows platinum hollow nanocages that exhibit electrocatalytic activity that is five times better than a standard platinum/carbon catalyst in an oxygen reduction reaction. (Figure courtesy of Georgia Tech.)
Xia says, “The net effect of this process is to take a nanosheet and fold it into a specific shape such as a cube.”
The researchers used density functional theory simulations to assist with determining what happens during the etching process. Xia says, “We believe that during the initial deposition of platinum atoms onto the substrate, some palladium atoms migrate into the platinum overlayers through intermixing. Once the etchant is applied, the palladium atoms are oxidized creating surface vacancies that eventually fuse into channels for the palladium atoms to diffuse to the surface from the template. The channels will enable the remaining palladium atoms to be removed by direct corrosion.”
Further analysis by scanning transmission electron microscopy shows that up to six atomic layers of platinum can form in the 100 orientation. Xia says, “The higher number of platinum atomic layers is due to interdiffusion of the palladium atoms into the platinum layers and an indication that the layers contain atomic vacancies that are not completely annealed out.”
The platinum hollow nanocages are evaluated as catalysts in an oxygen reduction reaction compared to a standard platinum/carbon catalyst. Octahedral platinum nanocages display electrocatalytic activity that is five times better than the standard catalyst. Xia says, “We synthesized octahedral platinum nanocages because they have a surface geometry that is more suitable for catalyzing oxygen reduction reaction. The hexagonal close-packed geometry leads to a twofold difference in catalysis compared to cubic nanocages.”
The nanocages displayed excellent durability after an evaluation for 10,000 cycles. A three- to four-fold enhancement in durability is seen compared to the conventional catalyst. Xia says, “The large hollow nanocage particle size relative to commercial platinum nanocatalysts (that are 2-3 nanometers in diameter) is the reason for the better durability. Catalytic activity is not lost because the larger hollow nanocage particles do not tend to aggregate.”
Future work will involve optimizing the platinum hollow nanocages. Xia says, “We will be trying to have a catalyst that is as thin as possible but still exhibits strong activity over a long operating time frame.”
Additional information can be found in a recent article (
2) or by contacting Xia at
younan.xia@bme.gatech.edu.
REFERENCES
1.
Canter, N. (2013), “Heterogenized homogeneous nanocatalysts,” TLT,
69 (2), pp. 12-13.
2.
Zhang, L, Roling, L., Wang, X., Vara, M., Chi, M., Liu, J., Choi, S., Park, J., Herron, J., Xie, Z., Mavrikakis, M. and Xia, Y. (2015), “Platinum-based nanocages with subnanometer-thick walls and well-defined, controllable facets,”
Science,
349 (6246), pp. 412-416.
 Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat can be submitted to him at neilcanter@comcast.net
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat can be submitted to him at neilcanter@comcast.net.