In fact, it’s the gas!
Drs. Wilfred T. Tysoe & Nicholas D. Spencer | TLT Cutting Edge October 2015
Elevated pressures of hydrogen or oxygen release adhesive junctions, thereby lowering friction in silicon oxide-doped, amorphous carbon coatings.

www.canstockphoto.com
AMORPHOUS HYDROGENATED CARBON (a-C:H)—an important type of diamond-like carbon (DLC)—films are attractive coatings since they can be easily applied as smooth, inert films that display low friction and wear. Although they are to be found in many applications that range from cutting tools to engines, they suffer from a number of problems, namely high residual stress (sometimes leading to delamination), low thermal stability and environment-dependent frictional behavior.
In an effort to modify these properties, other elements such as silicon and oxygen have been incorporated into a-C:H films. SiO-doped DLC (or a-C:H:Si:O) films, which reportedly consist of interpenetrating networks of silica and a-C:H, appear to be very promising for applications, showing lower residual stress and higher thermal stability than a-C:H. This latter property is thought to be related to the 4-coordinated Si atoms, which stabilize the carbon in its sp
3 (diamond-like) hybridization state, thereby inhibiting conversion to sp
2-hybridized (graphite-like) carbon at high temperatures.
It has been reported (
1) that the frictional properties of a-C:H:Si:O are less environment dependent than those of a-C:H, and that upon sliding, a transfer layer is formed on the steel countersurfaces. This tribofilm is carbonaceous in a dry environment—with a low-shear strength—but under humid conditions the shear strength increases and the film also contains silica fragments (
2). The effect of oxygen or hydrogen gas on the tribological behavior of a-C:H:Si:O had not been reported until a recent study by Koshigan et al., involving a collaboration between the University of Pennsylvania and the École Centrale de Lyon, France (
3). These authors used a host of analytical techniques, including scanning electron microscopy—with energy-dispersive x-ray analysis (EDX)—and near-edge x-ray absorption fine structure (NEXAFS) to understand the processes that had taken place at the a-C:H:Si:O steel interface during reciprocating sliding, both under vacuum, as well as in various pressures of oxygen and hydrogen.
The friction coefficient under vacuum or low gas pressures was extremely high (µ ≈ 1.2), but as the gas pressure was increased above 10 mbar for oxygen or 50 mbar for hydrogen there was a drastic decrease in the steady-state friction coefficient by at least an order of magnitude. EDX results showed that sliding under vacuum or low-pressure conditions resulted in an iron-containing transfer film appearing on the a-C:H:Si:O surface, while at high-gas pressure the tribofilm on the steel surface consisted only of C, O and Si. It can be concluded that the higher gas pressure appears to hinder adhesive junction growth to the steel and consequent transfer of the iron to the a-C:H:Si:O. Previous work for diamond- and hydrogen-free amorphous carbon films, for example, has shown that gases such as hydrogen and water can reduce friction by passivating dangling bonds formed during sliding and leaving them less reactive, preventing adhesive bonding and wear (
4). However, here the story is subtly different.
NEXAFS spectra of the worn region of the a-C:H:Si:O that had been exposed to higher gas pressures during sliding revealed the presence of extensive sp
2-hybridized carbon in the surface-near region. In the case of oxygen exposure, there also was evidence for the presence of carbon-oxygen bonds, presumably formed by O
2 dissociation and reaction with strained sp
2 C-C bonds. In the case of hydrogen, much less sp
2 carbon was detected following sliding than in the oxygen case, and no increase in C-H bonding was seen. This and the higher wear volume observed in hydrogen may suggest that the hydrogen etches the strained sp
2 C-C bonds to produce gas-phase hydrocarbons. This requires further confirmation.
The authors explain that in the absence of oxygen or hydrogen, the strained sp
2 C-C bonds react with the steel countersurface leading to adhesive-junction formation, iron transfer and high friction. In the presence of the gases, however, the C-C bonds break, allowing the transfer of the a-C:H:Si:O to the steel countersurface to leave behind a passivated sp
2 carbon surface (
see Figure 1). Thus this nice tribometry and surface-analytical study shows that in a-C:H:Si:O-steel sliding, material transfer occurs both under vacuum and at high-gas pressures but in opposite directions.
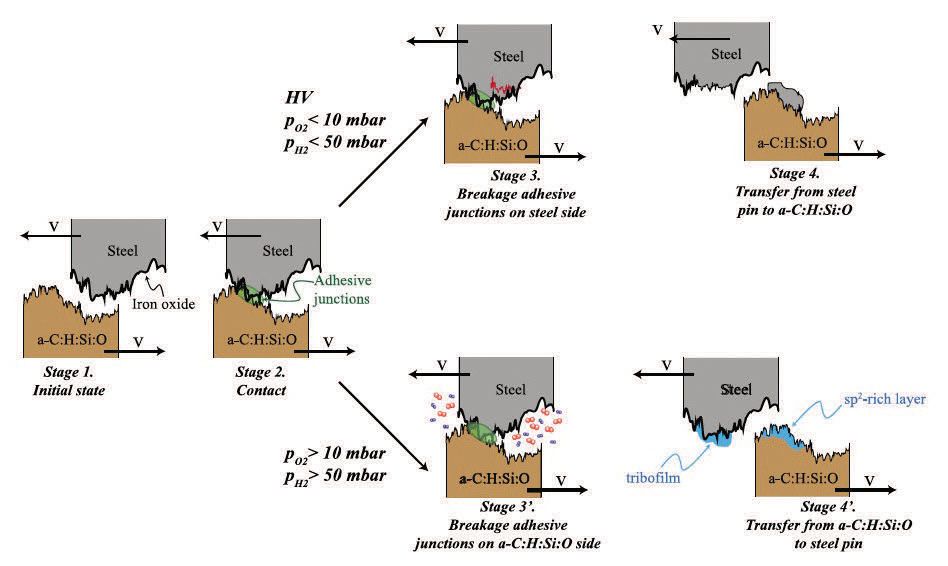 Figure 1. Schematic model for describing the friction properties of an a–C:H:Si:O versus steel tribosystem sliding under different environmental conditions (3).
REFERENCES
Figure 1. Schematic model for describing the friction properties of an a–C:H:Si:O versus steel tribosystem sliding under different environmental conditions (3).
REFERENCES
1.
Gilmore, R. and Hauert, R. (2001), “Control of the tribological moisture sensitivity of diamond-like carbon films by alloying with F, Ti or Si,”
Thin Solid Films,
398–399, pp. 199-204.
2.
Scharf, T.W., Ohlhausen, J.A., Tallant, D.R. and Prasad, S.V. (2007), “Mechanisms of friction in diamondlike nanocomposite coatings,”
J Appl. Phys.,
101, 063521-063521-11.
3.
Koshigan, K.D, Mangolini, F., McClimon, J.B., Vacher, B., Bec, S., Carpick, R.W. and Fontaine, J. (2015), “Understanding the hydrogen and oxygen gas pressure dependence of the tribological properties of silicon oxide–doped hydrogenated amorphous carbon coatings,”
Carbon,
93, pp. 851-860.
4.
Konicek, A.R., Grierson, D.S., Gilbert, P.U.P.A., Sawyer, W.G., Sumant, A.V. and Carpick, R.W. (2008), “Origin of ultralow friction and wear in ultrananocrystalline diamond,”
Phys. Rev. Lett.,
100, 235502/1-4.
 Eddy Tysoe is a Distinguished Professor of Physical Chemistry at the University of Wisconsin-Milwaukee. You can reach him at wtt@uwm.edu
Eddy Tysoe is a Distinguished Professor of Physical Chemistry at the University of Wisconsin-Milwaukee. You can reach him at wtt@uwm.edu.
 Nic Spencer is professor of surface science and technology at the ETH Zurich, Switzerland. You can reach him at nspencer@ethz.ch
Nic Spencer is professor of surface science and technology at the ETH Zurich, Switzerland. You can reach him at nspencer@ethz.ch.
Both serve as editors-in-chief of STLE-affiliated Tribology Letters journal.