Reaction to rubbing
Drs. Wilfred T. Tysoe & Nicholas D. Spencer | TLT Cutting Edge August 2015
Atomic force microscopy experiments reveal that interfacial shear can accelerate the rates of surface-chemical reactions.
THE RATES OF A CHEMICAL REACTION CAN BE ACCELERATED BY HEATING, and this is the most common strategy used by chemists to get a chemical reaction to go. However, reaction rates also can be increased by adding energy in the form of light, most notably by photosynthesis used by plants or by electrical energy during electrolysis.
In the context of tribology, it has been known for thousands of years that chemical reaction rates also can be accelerated by rubbing. Perhaps the earliest known example was provided by Theophrastus of Eresus, a student of Aristotle, in his treatise On Stones, written about 315 years B.C., in which mercury could be obtained by rubbing cinnabar (mercury (II) sulfide, HgS) mixed with vinegar in a brass mortar with a brass pestle.
Such shear-induced enhancements in chemical reaction rates are clearly relevant to tribochemistry but have always been difficult to measure because frictional heating, which always occurs during rubbing, also can accelerate chemical reaction rates. The obvious solution is to use an atomic force microscope (AFM) where the frictional heating is negligible, but unfortunately the contact area is generally so small that it is extremely difficult to analyze the surface during rubbing to measure the reaction rates. Two recent papers have shown how to overcome this problem.
In the first paper, Prof. Jonathan Felts from Texas A&M University, with Dr. Paul Sheehan and collaborators at the U.S. Naval Research Laboratory in Washington, D.C., studied shear-induced reactions of functional groups on hydrogenated, plasma-oxygenated and fluorinated graphene (
1). They realized that the friction of the sample varied linearly with the amount of adsorbate, thereby providing a direct
in situ method for monitoring the rate of the tribochemical reaction. As shown in Figure 1a, the friction, and hence the amount of adsorbate on the surface of oxygenated graphene, decreases exponentially with time (indicated as cumulative tip dwell time). The time to rub all of the molecules away also decreases as the tip load increases. By carefully calibrating their system and by measuring the contact area, they could plot the reaction rate as a function of contact stress as shown in Figure 1c.
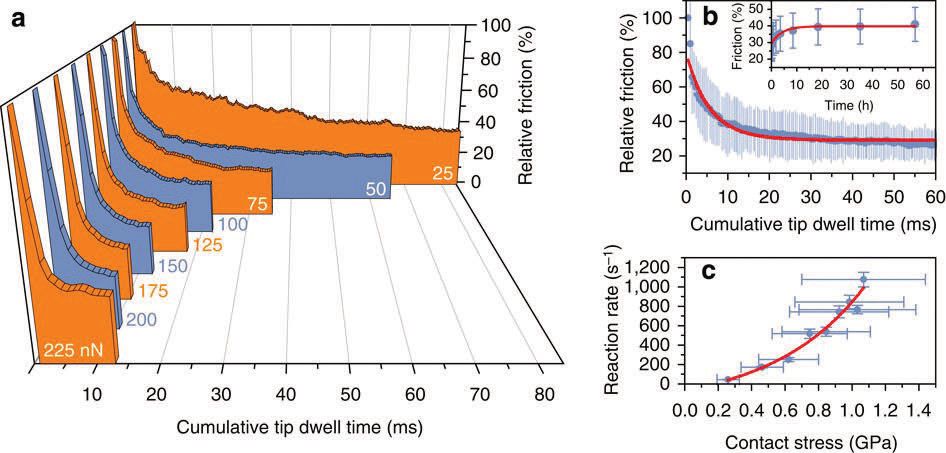 Figure 1. (a) Relative friction-force measurements as a function of cumulative tip dwell time for normal loads, showing an increasing, exponential decay rate with increasing normal load. (b) Reaction curve for 50 nN normal load showing that the kinetics follow an exponential decay typical of a first-order chemical reaction. At low loads, a contamination layer contributes an additional decay term. This contamination layer slowly re-adsorbs over many hours (see inset). (c) Reaction rate as a function of measured contact stress, showing an exponential dependence consistent with an atom-by-atom stress-assisted reaction mechanism. (Figure reproduced courtesy of Nature Communications (1).)
Figure 1. (a) Relative friction-force measurements as a function of cumulative tip dwell time for normal loads, showing an increasing, exponential decay rate with increasing normal load. (b) Reaction curve for 50 nN normal load showing that the kinetics follow an exponential decay typical of a first-order chemical reaction. At low loads, a contamination layer contributes an additional decay term. This contamination layer slowly re-adsorbs over many hours (see inset). (c) Reaction rate as a function of measured contact stress, showing an exponential dependence consistent with an atom-by-atom stress-assisted reaction mechanism. (Figure reproduced courtesy of Nature Communications (1).)
The rate increases exponentially with contact stress showing that tribochemical reactions can be induced just by sliding. They also were able to analyze their data to calculate the activation energy (without sliding) and found a value of 70 kJ/mol—typical of chemical reactions. The increase in reaction rate with contact stress could be characterized by a so-called “activation volume” where they found a value of ~11 Å
3, typical of the decomposition of CO-containing molecules.
In a second example, STLE-member Prof. Rob Carpick from the University of Pennsylvania, with collaborators from ExxonMobil, measured the rate at which a film grew from a common antiwear additive—zinc dialkyldithiophosphate (ZDDP) (
2). This is perhaps one of the most studied oil additives, but the film-growth pathways are still not very well understood.
As shown in Figure 2, the reaction rate could be simply measured by imaging the surface at a lower load and obtaining the film thickness directly. The results in Figure 2 show that the reaction rate increases exponentially with contact pressure, up to 5 GPa, similar to the results in Figure 1. At higher pressures, however, the rate became constant, and such behavior has been seen previously for antiwear films grown from ZDDP but not convincingly explained. Here it was found that the cessation of growth coincided with an increase in wear, as evidenced by the accumulation of material at the edge of the pads (
see Figure 2).
 Figure 2. Tribofilm volumetric growth rate dependence on contact pressure. The film growth rate is exponential at low contact pressures, but further growth is inhibited above ~5 GPa as the tip wears away newly deposited material. The 2 μm × 2 μm topographic contact-mode AFM images were acquired at a low load after generating tribofilms in the central 1.0 μm × 0.5 μm regions at various contact pressures. (Figure reproduced courtesy of Science Journal (2).)
Figure 2. Tribofilm volumetric growth rate dependence on contact pressure. The film growth rate is exponential at low contact pressures, but further growth is inhibited above ~5 GPa as the tip wears away newly deposited material. The 2 μm × 2 μm topographic contact-mode AFM images were acquired at a low load after generating tribofilms in the central 1.0 μm × 0.5 μm regions at various contact pressures. (Figure reproduced courtesy of Science Journal (2).)
Both of these sets of experiments clearly demonstrate the crucial role of shear stress in inducing tribochemical reactions. This will surely change the way in which we think about designing oil additives. Of course, frictional heating still occurs in real engineered systems, so the picture is likely to be much more complicated, but fundamental studies such as these will help us tease out the roles of temperature and contact stress on tribochemical reactions.
REFERENCES
1.
Felts, J.R., Oyer, A.J., Hernández, S.C., Whitener Jr., K.E., Robinson, J.T., Walton, S.G. and Sheehan, P.E. (2015), “Direct mechanochemical cleavage of functional groups from graphene,”
Nature Communications,
6, p. 6467.
2.
Gosvami, N.N., Bares, J.A., Mangolini, F., Konicek, A.R., Yablon, D.G. and Carpick, R.W. (2015), “Mechanisms of antiwear tribofilm growth revealed
in situ by single-asperity sliding contacts,”
Science,
348, p. 102.
 Eddy Tysoe is a Distinguished Professor of Physical Chemistry at the University of Wisconsin-Milwaukee. You can reach him at wtt@uwm.edu
Eddy Tysoe is a Distinguished Professor of Physical Chemistry at the University of Wisconsin-Milwaukee. You can reach him at wtt@uwm.edu.
 Nic Spencer is professor of surface science and technology at the ETH Zurich, Switzerland. You can reach him at nspencer@ethz.ch
Nic Spencer is professor of surface science and technology at the ETH Zurich, Switzerland. You can reach him at nspencer@ethz.ch.
Both serve as editors-in-chief of STLE-affiliated Tribology Letters journal.