Bouncing batteries
Dr. Neil Canter, Contributing Editor | TLT Tech Beat July 2015
An extensive evaluation has been conducted to see if there is a relationship between the health of the battery and how high it bounces.
KEY CONCEPTS
•
A battery’s health is usually determined through electrical testing, but bouncing the battery has become a popular alternative.
•
Researchers bounced hundreds of AA batteries and found that bouncing increases as the state of charge drops between 80% and 50%.
•
Zinc oxide formation through oxidation of the zinc anode causes the increase in bouncing, but this only tells the user that the battery is not fresh.
BATTERY PERFORMANCE OVER AN EXTENDED OPERATING TIME CONTINUES to be a focus of much research. Attention has mainly been placed on lithium-ion batteries because of their superior performance. Unfortunately, development of lithium-ion batteries has been hindered by concerns about their durability and flammability in use.
A previous TLT article revealed how researchers believe the instability in lithium-ion batteries is caused by the growth of metallic filaments known as dendrites as the battery ages. These dendrites grow initially at the anode into a fern-like structure that can eventually short circuit the battery (
1). Researchers prepared a new ion-conducting membrane based on aramid nanofibers and poly(ethylene oxide) that stops dendrite growth and improves durability while minimizing concern about failure.
A battery that most of us are more familiar with and use almost every day is the “alkaline” AA battery, also known as the LR6 form factor zinc-manganese dioxide battery. This battery type has been in use for more than 50 years and commands annual global sales of $1.8 billion.
Daniel Steingart, assistant professor of mechanical and aerospace engineering and the Andlinger Center for Energy and the Environment at Princeton University in Princeton, N.J., says, “Electrical testing is the main way to evaluate the health of a battery, but there are other non-electrical techniques that can be used to assess the chemistry of the battery. A favored approach is to analyze both the open circuit potential of the battery and the coulomb count, which measures how much charge is in the battery.”
Steingart points out that other factors—such as temperature rise—need to be evaluated in order to evaluate the safety limits in using the battery.
Bouncing has become a popular technique to determine whether an AA battery is dead. Steingart found out about this from a colleague. He says, “We were shown a video that demonstrates there can be a correlation between the ability of a battery to bounce and its performance.”
Steingart wanted a better understanding of whether bouncing a battery is a reliable technique for determining battery health and if the degree of bouncing provides any indication about the health of the battery.
Work has now been done to more systematically evaluate AA batteries.
COEFFICIENT OF RESTITUTION
Steingart and his colleagues have conducted an extensive evaluation of AA batteries to determine if there is a relationship between the health of the battery and how high it bounces. He says, “We evaluated a couple hundred batteries by dropping them through a 25 cm tall acrylic tube onto an epoxy benchtop and evaluating the audio to determine the number of bounces, height of the bounce and a term known as the coefficient of restitution (COR).”
COR refers to the ratio of the relative speed of an object after collision compared to before a collision. In the case of the collision between the battery and the epoxy benchtop, the COR refers to the square root of the height of one bounce to the earlier bounce.
Figure 1 shows an illustration of the testing done by Steingart’s research group. The state of charge of each battery was evaluated after each bouncing experiment. The battery was then discharged for one hour at 280 milliamps and retested. This process was repeated until the battery was completely discharged.
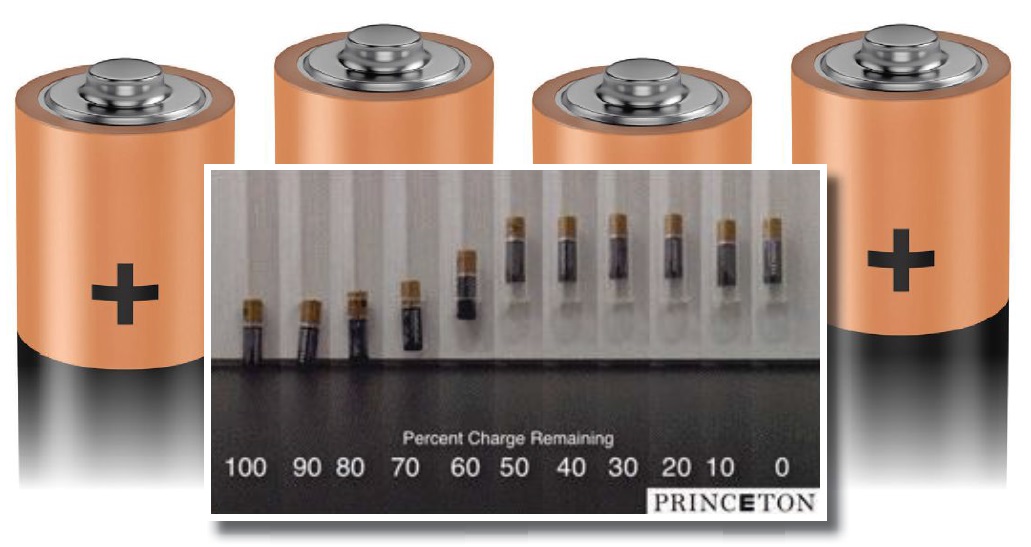 Figure 1. A recent study shows that the practice of bouncing batteries does not indicate if the battery is dead but does indicate that the battery is not fresh. (Figure courtesy of Princeton University.)
Figure 1. A recent study shows that the practice of bouncing batteries does not indicate if the battery is dead but does indicate that the battery is not fresh. (Figure courtesy of Princeton University.)
Data generated by the researchers shows that the COR of the batteries remains relatively stable until the state of charge drops to 80%. Then a dynamic increase in bouncing measured by COR takes place as the batteries state of charge drops to 50%. This is followed by the COR leveling off until the battery completely discharges.
The researchers hypothesized that the change in COR may be attributed to one of four effects including mass loss, reduction of the cathode, water consumption and oxidation of the zinc anode. Steingart says, “We evaluated each of these effects and concluded that the only one that is in agreement with the data is the oxidation of zinc to zinc oxide.”
Steingart continues, “As the battery discharges, zinc oxide forms through oxidation on the edges of the anode and eventually bridges develop between particles leading to the establishment of a network of springs that gives a battery the ability to bounce.”
This bridging effect was confirmed through the use of in situ energy-dispersive x-ray diffraction. Steingart also reveals that zinc oxide is used as a component to add bounce to golf balls.
The group hypothesizes that the COR levels off because the zinc oxide is more uniformly distributed through the anode and a second type of zinc oxide forms, which does not contribute to the bounce.
The important conclusion from this study is that bouncing a battery does not tell the user if it is dead but, rather, only that the battery is not fresh. Additional information about this work can be found in a recent study (
2).
Steingart indicates that follow-up work has focused on using sound to determine the state of charge in a battery. He says, “We have hooked up a speaker and a microphone to a battery to evaluate how the sound moves through a battery. A direct correlation is found between the state of charge in a battery and the density distribution as measured by sound.”
This procedure works well for any battery, including a lithium-ion battery. Further information can be found in a recent reference (
2) or by contacting Steingart at
steingart@princeton.edu.
REFERENCES
1.
Canter, N. (2015), “Dendrite-suppressing battery technology,” TLT,
71 (4), pp. 14-15.
2.
Bhadra, S., Hertzberg, B., Hsieh, A., Croft, M., Gallaway, J., Tassell, B., Chamoun, M., Erdonmez, C., Zhong, Z., Sholklapper, T. and Steingart, D. (2015), “The relationship between coefficient of restitution and state of charge of zinc alkaline primary LR6 batteries,”
J Mater. Chem. A,
3, pp. 9395-9400.
 Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat can be submitted to him at neilcanter@comcast.net
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat can be submitted to him at neilcanter@comcast.net.