Optimizing zeolite catalysts and sieves for base oil and biofuel production
Dr. Neil Canter, Contributing Editor | TLT Tech Beat June 2015
A computational screening process identifies new zeolites for petroleum oil processing and ethanol production.
KEY CONCEPTS
•
Zeolites are an important class of catalysts and sieves used in petroleum oil processing and the production of ethanol.
•
A multistep computational screening process has been developed to accelerate the identification of suitable zeolite candidates for use in both processes.
•
One of the top zeolites identified in the computational screening process was prepared, evaluated and found to perform very well in the purification of ethanol.
CATALYSTS AND SIEVES PERFORM A VERY IMPORTANT ROLE IN THE PROCESSING of base oils and in the purification of ethanol for use as fuel from fermentation broths. Production of Group II, Group III and GTL base oils has been expanding because these base stocks all are needed to ensure that high-performance lubricants—particularly those used in automotive applications—meet the more demanding requirements placed on them by OEMs.
One of the most important factors in today’s competitive environment is how to maximize the conversion of crude oil into these base stocks. Zeolite catalysts are widely used in this capacity in petroleum refineries.
Dr. Peng Bai, post-doctoral researcher at the University of Minnesota in Minneapolis, Minn., says, “Zeolites are used to sort, filter and trap chemical compounds and catalyze reactions needed to produce petroleum base oils. Currently, there are 225 known zeolites that are classified and given a three-letter code by the structure committee of the International Zeolite Association (IZA). Every year, about 10 more zeolites are discovered and need to be evaluated.”
In a previous TLT article, research describing the development of a new catalyst for use in isomerizing n-pentane to isopentane to boost the octane rating of gasoline is described (
1). The current process uses chlorinated alumina and zeolites as the catalysts in the refinery. A new zirconia-supported tungsten oxide nanocatalyst was discovered that provided good performance in this isomerization process. But the researchers doing this work took four years to develop and optimize the nanocatalyst.
A similar situation exists with production of ethanol for use as biofuel. Optimization of sorbent selectivity is critical to improve processing cost so that new approaches can enable ethanol production to be competitive with other fuels.
Bai says, “The traditional strategy for developing catalysts and sieves involves extensive experimentation, guided by often limited design principles. Our approach is to use computational modeling as a predictive tool to search the entire design space and discover potentially high-performing materials and then synthesize them and verify their performance through empirical work.”
Zeolite optimization can be better conducted through a predictive modeling process that can identify candidates that have a higher probability of providing specific catalytic or separation properties. Such a computational screening process has now been conducted to identify new zeolites for use in petroleum oil processing and production of ethanol.
MULTISTEP COMPUTATIONAL SCREENING
The team at the University of Minnesota—in collaboration with a researcher at Rice University—used a supercomputer at Argonne National Laboratory to do a multistep computational screening of both known and predicted zeolites to identify suitable candidates for specific steps in the production of base oil and ethanol. The team says, “We expanded our candidates by including over 300,000 zeolites that are predicted to be thermodynamically accessible. This gave us an opportunity to find both materials that already exist and those that can potentially be synthesized.”
The objective of the zeolite screening for use in base oil manufacture was to find candidates that can be effective in the isomerization of straight chain hydrocarbons ranging from 18-30 carbon atoms to slightly branched hydrocarbons. Bai says, “Bifunctional zeolites, when combined with suitable embedded Group VIII metal, can all conduct this isomerization process. The question is whether it will dominate versus other reactions such as cracking and oligomerization, which we want to minimize and whether the desired products will be produced quickly and selectively over other byproducts.”
In ethanol production, one of the most troublesome steps is separation from water. Bai says, “We are looking to determine if a zeolite can have both the adsorption selectivity and capacity to separate ethanol from water. High selectivity means that ethanol will be predominantly adsorbed by the sorbent leaving water in the fermentation broths.”
The researchers assessed the zeolites and ranked them for both applications through a performance score as shown in Figure 2. Of the top 10 zeolite sieves and catalysts from the IZA database identified in both applications, three are new ones for isomerization of base oils while all are new ones suitable for separating ethanol from water. For comparison, the data in Figure 2 also includes some hypothetical zeolites that are predicted to perform exceedingly well for the isomerization and zeolite MFI that is currently used for the ethanol purification.
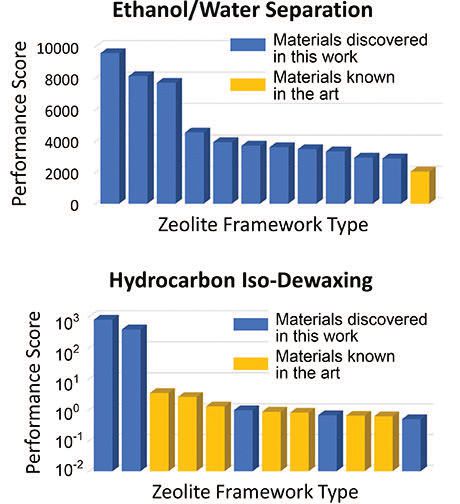 Figure 2. After reviewing a database of over 300,000 known and predicted zeolites, the top catalysts for use in ethanol/water screening and in hydrocarbon iso-dewaxing are shown. (Figure courtesy of the University of Minnesota College of Science and Engineering.)
Figure 2. After reviewing a database of over 300,000 known and predicted zeolites, the top catalysts for use in ethanol/water screening and in hydrocarbon iso-dewaxing are shown. (Figure courtesy of the University of Minnesota College of Science and Engineering.)
Bai says, “We then prepared and evaluated one of the top zeolites predicted to be highly selective in the purification of ethanol. The results indicate that the material indeed performs very well and could help to improve the efficiency of the separation. But synthesizing zeolites in the desired form for a given application is often a lengthy and laborious process, and this experimental verification alone took three months. Considering the huge number of zeolites that can be made, it highlights that our approach to narrow down the list of potentially attractive targets through the multistep computational screening process would be very helpful in reducing experimental efforts and speeding up materials discovery.”
Future research will utilize the computational modeling for other separation and catalysis problems and to develop better strategies for synthesizing zeolites. Bai says, “At the University of Minnesota, we are extending this high-throughput screening approach to separations encountered in biomass conversion and petroleum refining and for applications involving detection or capture of specific compounds. At Rice University, researchers are exploring computational methods to optimize structure-directing agents that can promote the growth of a given zeolite structure.”
Additional information can be found in a recent article (
2) or by contacting Professor Ilja Siepmann at
siepmann@umn.edu.
REFERENCES
1.
Canter, N. (2010), “Nanocatalyst for refinery use,” TLT,
66 (12), pp. 12-13.
2.
Bai, P., Jeon, M., Ren. L., Knight, C., Deem, M., Tsapatsis, M. and Siepmann, I. (2015), “Discovery of optimal zeolites for challenging separations and chemical transformations using predictive materials modeling,”
Nature Communications,
6, 5912, DOI: 10.1038/ncomms6912.
 Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat can be submitted to him at neilcanter@comcast.net
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat can be submitted to him at neilcanter@comcast.net.