Pitting corrosion model
Dr. Neil Canter, Contributing Editor | TLT Tech Beat June 2015
A new modeling strategy accounts for the movement of all metal ions during pitting corrosion.
KEY CONCEPTS
•
A new model for pitting corrosion accounts for the movement of metal ions not only through the aqueous phase but also through the solid metal.
•
Two processes referred to as corrosion-dependent damage and damage-dependent corrosion are used in developing the model.
•
The model shows the rate of pitting corrosion increases in the much less corrosion-resistant area underneath the metal surface.
CORROSION CONTINUES TO BE A CHALLENGING PROBLEM BECAUSE OF THE DAMAGE it can inflict on machinery during use, creating operating problems that limit performance. The annual cost of corrosion to the global economy is immense.
From a lubricant perspective, many different types of corrosion inhibitors have been developed to deal with the problem. In a previous TLT article, research describing the benefits of graphene as a corrosion inhibitor was described (
1). Application of multiple layers of graphene slows down the rate of corrosion on copper and nickel by seven and 20 times, respectively, compared to the bare metals.
Pitting corrosion originates in a specific location and forms localized holes in the metal surface. In many cases, it is very difficult to detect, making it a significant threat to the long-term viability of the metal.
The traditional way to consider pitting corrosion is by the exposure of a metal surface to an aqueous environment. An electric circuit is established where metal ions are oxidized at an anode releasing electrons that are consumed at a cathode.
Florin Bobaru, professor of mechanical and materials engineering at the University of Nebraska-Lincoln in Lincoln, Neb., says, “Past modeling of pitting corrosion has focused on effects occurring at the interface between the metal surface and the aqueous environment. Most of these models impose too many specific conditions and as a result are too restrictive in explaining how boundary conditions change. But this modeling does not consider that the metal surface is in fact rough and contains corrosion processes that take place through a significant subsurface layer.”
Past pitting corrosion models do not take into account that metal ions move not just into the aqueous phase but also through the solid metal when the corroding solution “wets” a layer of a certain thickness beneath the pit surface. A new modeling strategy is needed to account for the movement of all metal ions during pitting corrosion. This dislocation of metal ions leads to material degradation, further introducing spots where stress concentrations can lead to initiation and propagation of catastrophic cracks. Such a model has now been developed.
PERIDYNAMIC APPROACH
Bobaru and his colleague, post-doctoral research associate Ziguang Chen, have developed a new model for pitting corrosion that computes the evolution of the pit shape and of damage induced by corrosion through a subsurface layer within the metal. Bobaru says, “Our model is a peridynamic one, which means that it evaluates not just the nearest neighbors of a specific location but also those that are nearby. The non-local interactions are important in modeling unrestricted evolution of damage.”
The researchers focused on the metal oxidation occurring at the anode because this is the most important factor in controlling the rate of damage caused by pitting corrosion. Bobaru says, “We looked at published empirical research on pitting corrosion in developing the parameters of the model to ensure that the actual results seen are simulated properly. The key factor is to determine how the interface between the corroding surface and the metal surface changes over time once pitting corrosion has started.”
Two processes referred to as corrosion-dependent damage and damage-dependent corrosion are used in developing the model in one dimension and then expanding it to two and three dimensions. Bobaru says, “Corrosion-dependent damage occurs when sufficient metal ions leave a specific area of the metal causing additional metal bonds to break, leading to damage to the integrity of the metal. When damage reaches a certain critical value, it is reasonable to assume a phase-change at the location (the metal particle has dissolved in the solution), which accelerates the diffusion of metal ions. We make the assumption that fresh liquid constantly flushes the corrosion damage surface leading to rapid movement of metal ions. This assumption is consistent with what is occurring in real world applications.”
Figure 1 shows snapshots from the model detailing how corrosion starts on the metal surface and then moves into the subsurface. Corrosion becomes more damaging in moving from a blue color, which is a pristine metal, to a red color, which represents completely damaged/dissolved material. The colors between blue and red correspond to partially damaged material.
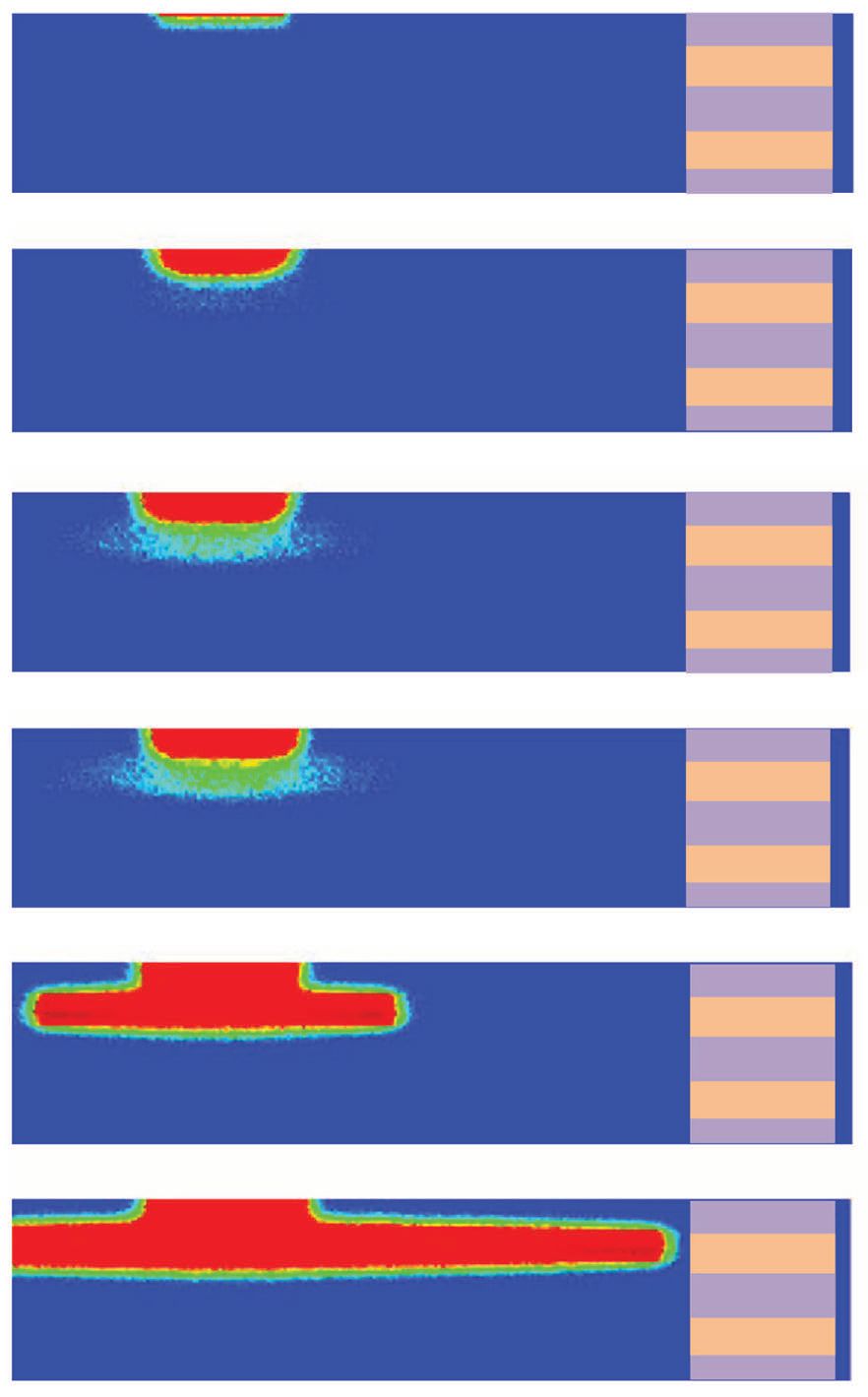 Figure 1. Snapshots from a new pitting corrosion model show how corrosion starts on the metal surface and then moves to the subsurface. The red color prominently seen in the subsurface is an indication that this is the area where corrosion-resistance is weaker in a metal as compared to the surface. (Figure courtesy of the University of Nebraska-Lincoln.)
Figure 1. Snapshots from a new pitting corrosion model show how corrosion starts on the metal surface and then moves to the subsurface. The red color prominently seen in the subsurface is an indication that this is the area where corrosion-resistance is weaker in a metal as compared to the surface. (Figure courtesy of the University of Nebraska-Lincoln.)
Bobaru says, “For most metals, the surface is the location where corrosion-resistance is highest. Our model shows that once pitting corrosion is present, the rate increases in the much less resistant underneath.”
This current model means that the extent of pitting corrosion in a specific location cannot be determined just by monitoring the metal ion concentration in the liquid phase. Ion concentration (and damage caused by corrosion) must also be determined on the metal surface and in the subsurface.
Future work will involve modeling stress corrosion cracking. Bobaru says, “We believe that our model for corrosion damage can now be coupled with our earlier mechanical crack-growth model for analysis of stress corrosion cracking causes of structural failure. Our objective is to develop a model to explain how this failure takes place supported by experimentation.”
Additional information can be found in a recently published article (
2) or by contacting Bobaru at
fbobaru2@unl.edu.
REFERENCES
1.
Canter, N. (2012), “Graphene: Potential corrosion inhibitor,” TLT,
68 (7), pp. 12-13.
2.
Chen, Z. and Bobaru, F. (2015), “Peridynamic modeling of pitting corrosion damage,”
Journal of the Mechanics and Physics of Solids,
78, pp. 352-381.
 Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat can be submitted to him at neilcanter@comcast.net
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat can be submitted to him at neilcanter@comcast.net.