Vulnerability of titanium to oxygen
Dr. Neil Canter, Contributing Editor | TLT Tech Beat May 2015
New research reveals why oxygen causes titanium’s physical properties to decline.
KEY CONCEPTS
•
Very low concentrations of oxygen in titanium can cause the metal to harden and become more brittle.
•
This effect occurs because one dislocation in the titanium interacts with an oxygen atom to form multiple dislocations.
•
While the strength of titanium can increase by a factor of three due to multiple dislocations, this will eventually cause titanium's resistance to cracking to decline by six times.
WITH THE CONTINUING TREND TO FIND APPROACHES TO INCREASE THE EFFICIENCY OF MACHINERY, lightweight metals are clearly being looked at more closely as a substitute for ferrous alloys. Metals such as titanium offer a superior strength-to-weight ratio and better resistance to corrosion than steel. But titanium is also more expensive.
In a previous TLT article, a new approach was made to reduce the cost of titanium through a potentially lower-cost manufacturing pathway using titanium hydride (
1). The existing method for extracting titanium is known as the Kroll process, which is conducted through a series of steps at elevated temperatures in excess of 980 C. Use of titanium hydride led to the formation of titanium in modeling studies that involved the use of much lower temperatures, thereby leading to the promise of a lower-cost, more energy efficient process.
One of the problems in finding a better way to produce titanium is oxygen. Andrew Minor, associate professor of materials science and engineering at the University of California in Berkeley, Calif., and faculty scientist at Lawrence Berkeley National Laboratory, says, “Very low concentrations of oxygen in titanium leads to the hardening of the metal, which eventually causes a decrease in toughness in the titanium metal that makes it less resistant to cracks.”
A better understanding of how oxygen causes titanium’s physical properties to decline is needed in order to determine what can be done to figure out a more cost effective method for extracting and processing titanium. Research has now been done showing what oxygen does to titanium on the atomic level.
DISLOCATION MULTIPLIER
Minor and his associates at the Berkeley Laboratory, in collaboration with colleagues at Japan’s Nuclear Science and Engineering Directorate and Rolls Royce (a manufacturer of jet turbine engines), has determined how oxygen at very low concentrations will cause titanium to become harder and then eventually more brittle. The presence of oxygen in titanium is similar to metals containing other components that act as solutes to improve mechanical properties, but the effect is far more extreme than normal solute hardening.
Minor says, “Solutes are added to every metal to make alloys. One of the main reasons for doing this is to increase the hardening of the resulting metallic alloys. This process takes place because the solutes are atoms with a different size than the plane of metallic atoms they are encountering. Solutes end up being attracted to dislocations, which represents extra spaces in the metal for foreign atoms that do not fit perfectly within the matrix lattice.”
An analogy to this process as explained by Minor is moving a rug along a floor by applying a ripple (the dislocation) that flows through the fiber of the rug. There are two types of dislocations seen in metals. One is called an edge dislocation while the second one is known as screw dislocation. Both are equivalently missing half-planes of atoms in the crystal structure of a material.
Minor explains that the solute atoms are typically either placed in a substitution manner in the same plane as the metal atoms or in an interstitial fashion in between the metal atom planes.
The researchers studied the alpha form of titanium, which is readily available commercially, doped with oxygen at concentrations of 0.1%, 0.2% and 0.3% by weight using a technique known as aberration-corrected transmission electron microscopy (TEM) to determine the effect of the oxygen atoms on the titanium metal matrix over time.
Minor says, “Oxygen generates a very strong negative repulsion with the screw dislocations in titanium at a very small concentration. Normally, for most alloys, a solute concentration between 0.1%-0.3% does not make much difference in the resulting properties. But with titanium, the difference between three and one oxygen atoms within 1,000 titanium atoms will cause a very different motion of the dislocations.”
The TEM data shows that dislocation in titanium interacts with an oxygen atom (
shown in red in Figure 1) to generate additional dislocations. Minor says, “This oxygen atom acts as a multiplier to produce additional dislocations leading to more tangling of the dislocations and therefore results in the titanium becoming more brittle.”
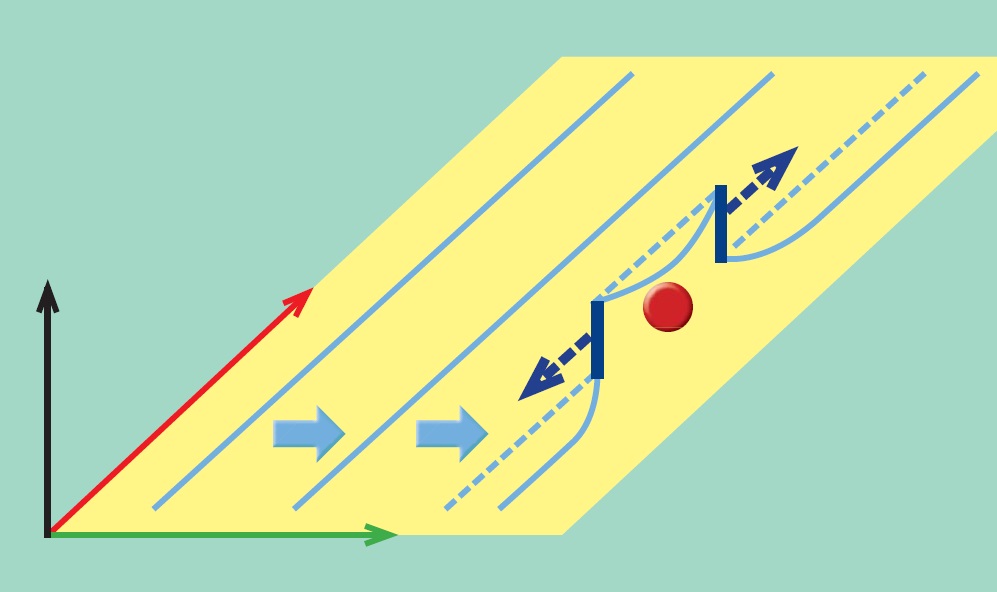 Figure 1. A moving dislocation (shown in light blue) in titanium can interact with an oxygen atom (shown in red) to form multiple dislocations (shown in dark blue) leading titanium to be much less resistant to cracking. (Figure courtesy of the University of California Berkeley.)
Figure 1. A moving dislocation (shown in light blue) in titanium can interact with an oxygen atom (shown in red) to form multiple dislocations (shown in dark blue) leading titanium to be much less resistant to cracking. (Figure courtesy of the University of California Berkeley.)
The researchers used
in situ TEM nanocompression testing to assess the impact of these dislocations in real time on the mechanical properties of titanium. Minor says, “We found that the strength of titanium increases by a factor of three due to the multiplier dislocation effect of oxygen atoms—this leads to the effect of six times less resistance to cracking.”
Future work will concern finding techniques for preventing oxygen atoms from causing titanium to become brittle. Minor says, “We will try to use computational means to determine how to lock up oxygen atoms to mitigate this effect, such as finding elements that can bind with the oxygen preventing the dislocation multiplier effect.”
The hope of the researchers is that this work will help in the development of a lower-cost process for refining titanium without the metal losing its optimal characteristics. Additional information can be found in a recent article (
2) or by contacting Minor at
aminor@berkeley.edu.
REFERENCES
1.
Canter, N. (2014), “Cost-effective titanium extraction,” TLT,
70 (7), pp. 12-13.
2.
Yu, Q., Qi, L., Tsuru, T., Traylor, R., Rugg, D., Morris, J., Asta, M., Chrzan, D. and Minor, A. (2015), “Origin of dramatic oxygen solute strengthening effect in titanium,”
Science,
347 (6222), pp. 635-639.
 Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat can be submitted to him at neilcanter@comcast.net
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat can be submitted to him at neilcanter@comcast.net.