Controlling friction at the nanoscale
Dr. Neil Canter, Contributing Editor | TLT Tech Beat April 2015
Application of an electric field and adjustment of the relative humidity can be used to control friction.
KEY CONCEPTS
•
Implementation of an electric field and adjustment of the relative humidity of the environment can adjust the nanoscale friction of ionic solids.
•
Water solubility of the ionic salt is important because friction reduction can occur at a low relative humidity for those salts with high solubility in water.
•
The mechanism for this friction reduction is due to the formation of an electric double layer consisting of layers of cations and anions from a specific salt at the junction of the AFM tip and the surface of the salt.
ACHIEVING A BETTER UNDERSTANDING OF FRICTION AT THE NANOSCALE is becoming increasingly important as new technologies such as microelectromechanical systems are under development. Research has shown that friction at the nanoscale, which evaluates the interaction of atoms with each other, is different from how two surfaces contact each other at the macroscale.
In a previous TLT article, research evaluating the friction measured when a single polymer chain is pulled off a surface was described (
1). This was accomplished by attaching the polymer chain to the cantilever tip of an atomic force microscope (AFM) and then retracting the tip more than 100 nanometers from the surface.
During the process, the researchers in this study found that a third friction mechanism known as desorption stick is present along with slip and cooperative stick. This third mechanism was discovered because the researchers were able to utilize the more sensitive vertical signal of the AFM to decouple frictional force from desorption force.
The efforts to continue to find ways to reduce friction have led Evgheni Strelcov, post-doctoral research associate in the Center for Nanophase Materials Sciences (CNMS) at Oak Ridge National Laboratory (ORNL) in Oak Ridge, Tenn., to determine what happens when an electric field is applied. He says, “In earlier research in our group at CNMS, application of an external electric field led to an electromechanical response in the material, allowing probing ferroelectric polarization switching and electrochemical reactivity. I was curious to see what applications of an electric field can do for a different functionality, such as friction, and different systems, including soft matter.”
This vision inspired Strelcov and his colleagues at ORNL—Wigner Fellow Vera Bocharova, staff scientist Rajeev Kumar and senior researchers Bobby Sumpter and Sergei Kalinin—to start working with polymerized ionic liquids that exhibit low melting points, low glass transition temperatures and high ionic mobility. Strelcov says, “Unexpectedly, application of an electric field caused the polymerized ionic liquid to soften and almost melt. When an AFM was used, the tip moved easily into the material—almost like a hot knife through butter.” In addition, the AFM showed dramatic changes in friction forces acting on a scanning tip, suggesting intrinsic connection between these two phenomena.
The initial thought was the electric field caused a local lowering of the melting point of the polymer. To verify his hypothesis, Strelcov initiated systematic studies of a broad range of materials. Further work with sodium thiosulfate showed the same effect. Strelcov says, “We chose sodium thiosulfate because it has a low melting point (48 C) relative to most ionic solids.”
The softening of the ionic solids suggests that friction is decreasing because the cantilever tip can more easily slide on the surface. Additional research was done with high-temperature ionic solids and the researchers that saw this “softening effect” also leads to a reduction in friction. Strelcov says, “Based on the work done with the high temperature ionic solids, we know that the softening is not occurring due to melting. One other factor is that this phenomenon could only occur if there was moisture present. At a relative humidity of 0%, nothing happened. At higher humidity, both softening and a reduction in friction were observed.”
Further work by Strelcov and his colleagues has now shown that nanoscale friction of ionic solids on the nanoscale can be adjusted through the implementation of an electric field and adjusting the relative humidity of the environment.
ELECTRIC DOUBLE-LAYER MECHANISM
Strelcov and his colleagues conducted a systematic study to evaluate how friction changes when a conductive AFM tip interacts with the surface of various salts while varying the voltage of an applied electric field and adjusting the relative humidity of the environment. Figure 1 shows how the AFM tip interacts with the surface of a salt.
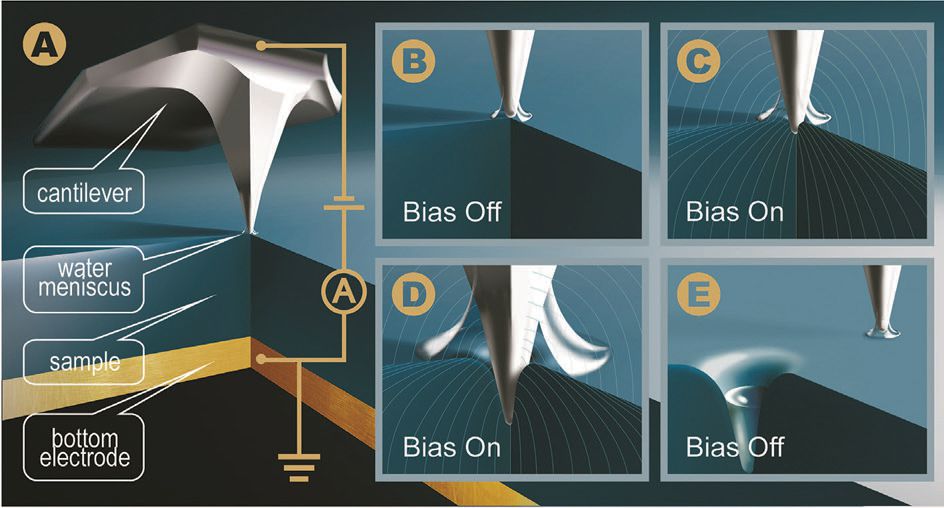 Figure 1. The interaction of the AFM tip with the surface of an ionic salt shows how friction can be tuned at the nanoscale. (Figure courtesy of Oak Ridge National Laboratory.)
Figure 1. The interaction of the AFM tip with the surface of an ionic salt shows how friction can be tuned at the nanoscale. (Figure courtesy of Oak Ridge National Laboratory.)
The technique used by the researchers is known as the first order reversal curve current-voltage topography spectroscopy and is shown in Figure 1(a). At a specific spot above the salt surface, a voltage bias (degree of voltage applied) moves from zero to a maximum value and the researchers determined how the AFM tip moved into the salt surface. Figure 1(b) shows the AFM tip at the beginning of the process before an electric field is applied, Figure 1(c) shows the AFM tip as it starts to move into the salt surface, Figure 1(d) shows that the AFM tip penetrates into the salt surface and Figure 1(e) shows that the AFM tip has moved to another location after the electric field was turned off.
The researchers evaluated a series of inorganic salts including those derived from alkali and alkali earth metals. Strelcov says, “We evaluated salts of potassium, sodium, rubidium, cesium, magnesium and aluminum including sodium chloride, sodium sulfate, potassium nitrate, magnesium sulfate, aluminum sulfate, etc.” An important aspect of such nanoscale friction tuning is that it does not require flow current, thus eliminating electrical power losses in parasitic processes, such as Joule heating.
In varying the electric field and the relative humidity, the researchers determined that a relationship exists between the water solubility of the salt and changes in friction in response to an applied electric field at a given relative humidity. Strelcov says, “For ionic salts with high solubility in water, we found that friction reduction can occur at a low relative humidity. With ionic salts that are not very soluble in water, the relative humidity needed to be higher to produce a reduction in friction.”
Friction tuning can vary depending upon the salt, the magnitude of the voltage applied and the relative humidity. Some ionic salts did not show any effect. Strelcov says, “We studied cobalt bromide and could not see any friction reduction because this salt is very hygroscopic and its surface is unstable at the nanoscale.”
One other factor is molecular solids that are both water soluble (such as sucrose and urea) and insoluble (such as polystyrene and quartz) do not show any frictional change. Strelcov says, “We observed a difference in the surface topography when the AFM tip encountered the salt surface based on whether friction increased or decreased. The topography changed significantly when the friction increased but remained unchanged when the friction decreased.”
To explain this frictional change, Strelcov and his colleagues are proposing that the reduction in friction is caused by the formation of an electric double layer. Strelcov says, “We believe that an electric double layer consisting of layers of cations and anions from a specific salt is formed at the junction of the AFM tip and the surface of the salt. As voltage is applied to the AFM tip, water vapors condense in a very strong electric field at the tip-surface junction, dissolving the salt locally and moving the tip into the surface. The simultaneously generated electric double layer facilitates the formation of a hole that is orders of magnitude higher (from 100 nanometers up to 1.5 microns in diameter) than the diameter of an AFM (20-50 nanometers). The formed solution is expelled from the vicinity of the tip-surface junction (away from the strong electric field) along the surface followed by replacement of fresh water from the vapor phase.”
This expulsion is consistent with a thermodynamic force that causes a desired minimization of free energy.
Future work will involve further clarification of the mechanism. Strelcov says, “We are still working to try and develop a model to explain this effect.”
The interesting aspect of this work is that water, which has traditionally been a poor lubricant, can interact with ionic salts to reduce friction at the nanoscale. Additional information can be found in a recent article (
2) and by contacting Strelcov at
strelcove@ornl.gov.
Note: This research was conducted at the CNMS, which is a DOE Office of Science User Facility. Vera Bocharova would like to acknowledge sponsorship by the Laboratory Directed Research and Development Program of ORNL, managed by UT-Battelle, LLC, for the U.S. DOE.
REFERENCES
1.
Canter, N. (2013), “New type of friction observed at the nanoscale,” TLT,
69 (8), pp. 10-11.
2.
Strelcov, E., Kumar, R., Bocharova, V., Sumpter, B., Tselev, A. and Kalinin, S. (2015), “Nanoscale lubrication of ionic surfaces controlled via a strong electric field,”
Scientific Reports,
5 (8049), DOI: 10.1038/srep08049.
 Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat can be submitted to him at neilcanter@comcast.net
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat can be submitted to him at neilcanter@comcast.net.