Frictional properties of fluorinated graphene at the nanoscale
Dr. Neil Canter, Contributing Editor | TLT Tech Beat February 2015
The difference in friction forces is due to a property known as energy corrugation.
KEY CONCEPTS
•
Graphene was fluorinated to various degrees enabling the creation of fluorinated regions next to pristine regions.
•
Evaluation of the frictional interactions between fluorinated graphene and a silicon AFM tip showed that friction forces are more than 10 times higher for fluorinated graphene compared to pristine graphene.
•
This difference in friction is due to an increase in the electronic roughness seen with fluorinated graphene compared to pristine graphene.
GRAPHENE, A TWO-DIMENSIONAL SHEET containing carbon atoms organized into a hexagonal pattern that has the appearance of a honeycomb lattice, has been under extensive evaluation since it was first produced in a lab in 2004. This material has been the subject of several TLT articles, including detailing work done showing it is the strongest material ever examined (
1).
The reason for the attention received by graphene is that this material will significantly reduce friction at both the nanoscale and the macroscale. STLE-member Robert Carpick, John Henry Towne Professor and chair of mechanical engineering and applied mechanics at the University of Pennsylvania in Philadelphia, discusses the current understanding researchers have about graphene. He says, “Just a few layers or even one layer of graphene have been shown to greatly reduce the frictional forces seen between nanoscale tips and surfaces such as copper, silicon, silicon dioxide, nickel and silicon carbide.”
A previous TLT article highlighted work done by Carpick that showed the friction between the tip of an atomic force microscope (AFM) and graphene increases as the thickness of graphene layers declines (
2). An estimate was made that the friction increased by two to three times in moving from a bulk sheet of graphene atoms to a monolayer and by 20 percent in moving from a bilayer of atoms to a monolayer. The explanation for this effect is that a single layer of graphene conforms more readily to the AFM tip than multiple layers, leading to larger contact area and higher friction.
The challenge in applying these results to real applications, according to Carpick, is graphene’s durability. He asks, “How long will graphene last before the layers wear away causing friction to rise?”
A new approach is needed to find a way to improve the low frictional characteristics of graphene at the nanoscale. One thought is graphene derivatives.
STLE-member Xin Liu, a doctoral student at the University of Pennsylvania, says, “Polytetrafluoroethylene displays much better frictional properties than graphite in a humid environment. This suggest that a two-dimensional analogue of polytetrafluoroethylene should be studied to see if fluorination will lead to improved frictional properties at the nanoscale.”
ENERGY CORRUGATION
Collaborators Jeremy Robinson and Paul Sheehan at the Naval Research Laboratory (NRL), fluorinated graphene to various degrees, and Carpick and Liu studied the effects of this derivatization on the coefficient of friction at the nanoscale. Xin says, “Graphene films on a copper substrate were prepared by NRL through chemical vapor deposition. They were then transferred onto a silicon dioxide substrate and coated with a photoresist layer to protect parts of the graphene. The samples were then exposed to xenon difluoride gas for different time periods, creating fluorinated regions next to pristine regions that had been protected by the photoresist.”
This procedure led to the preparation of locally fluorinated graphene and enabled the researchers to directly compare this material with graphene side by side. Evaluation of the frictional interactions between monolayer fluorinated graphene and the silicon AFM tip shows that the friction forces are much higher, even more than 10 times, for fluorinated graphene as compared to pristine graphene.
The reason for this difference in friction forces is due to a property known as energy corrugation. Carpick says, “The corrugation is a physical term that describes the potential energy range from a high point to a low point between the AFM tip and the sample. There is a direct correlation between corrugation and friction, as initially outlined by the models developed by Prandtl and by Tomlinson in the 1920s.”
The corrugation can be thought of as the electronic topography or the hills and valleys of the electron density at the surface. For pristine graphene, the electron density is very flat, so there are only very shallow valleys of electronic potential energy between the high points, as shown in the top graph of Figure 2. However, the presence of fluorine atoms in fluorinated graphene leads to a far more corrugated charge distribution.
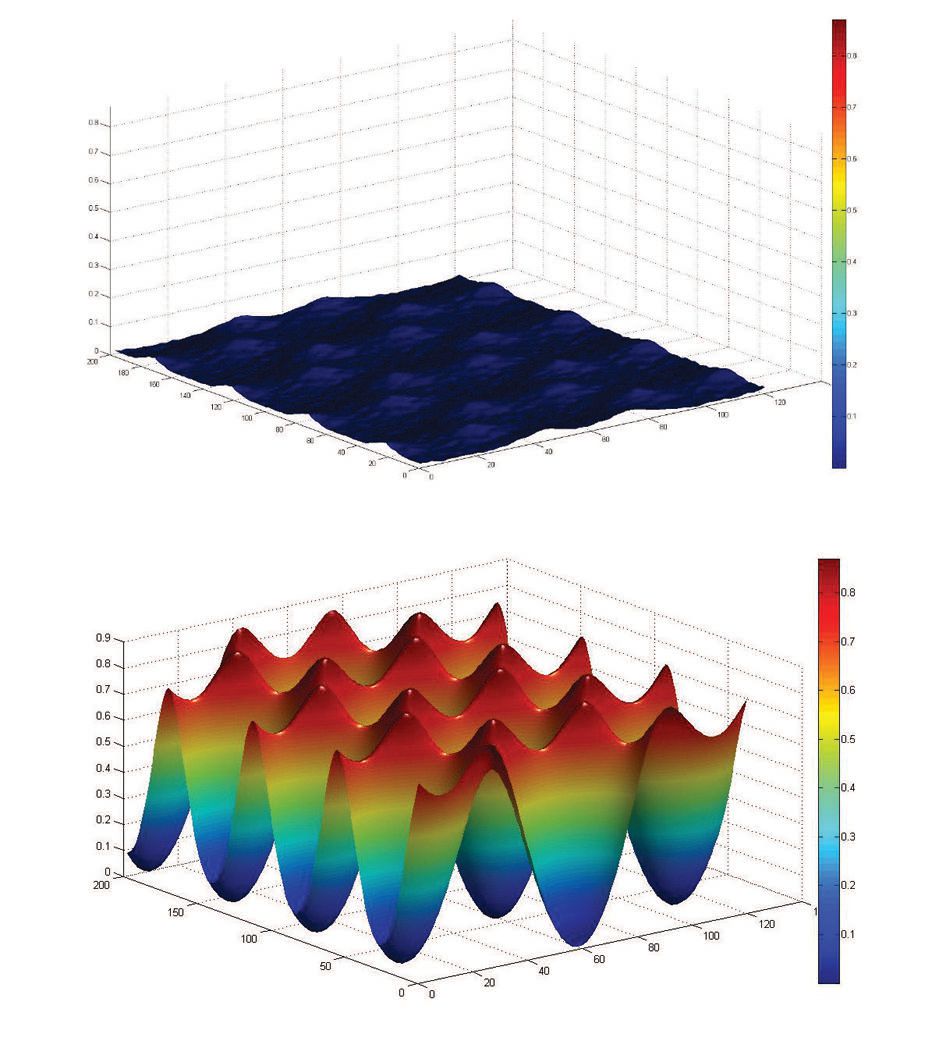 Figure 2. The difference in the electronic potential energy of pristine graphene (top) and fluorinated graphene (bottom) explains why the friction forces are more than 10 times higher for fluorinated graphene compared to pristine graphene. Figure courtesy of the University of Pennsylvania.
Figure 2. The difference in the electronic potential energy of pristine graphene (top) and fluorinated graphene (bottom) explains why the friction forces are more than 10 times higher for fluorinated graphene compared to pristine graphene. Figure courtesy of the University of Pennsylvania.
According to molecular dynamics (MD) simulations, performed in collaboration with the Shenoy Research Group at the University of Pennsylvania, there is a high concentration of negative charge over the fluorine atoms because of the high degree of electronegativity of this atom. The high deficiency of charge in between the fluorine atoms leads to significant peaks and valleys in the energy landscape of fluorinated graphene, as shown in the bottom graph of Figure 2. This increase in the electronic roughness seen with fluorinated graphene as compared to graphene is central in explaining why the friction increases.
Carpick says, “The much higher level of corrugation in fluorinated graphene directly leads to a higher level of friction as compared to graphene. From the MD results, we estimated that energy corrugation is higher in fluorinated graphene by a factor between 10 and 20 so friction is also higher by that same magnitude.”
The results seen by the researchers are consistent with the Prandtl-Tomlinson model. Future work will involve evaluating the impact of other functionalities on graphene and also evaluating other solid lubricants at the nanoscale such as molybdenum disulfide.
Further information can be found in a recent article (
3) or by contacting Carpick at
carpick@seas.upenn.edu.
REFERENCES
1.
Canter. N. (2009), “Graphene: The Strongest Material ever Examined,” TLT,
65 (2), pp. 28-29.
2.
Canter, N. (2010), “Size does matter for nanoscale friction,” TLT,
66 (8), pp. 10-11.
3.
Li, Q., Liu, X., Kim, S., Shenoy, V., Sheehan, P., Robinson, J. and Carpick, R. (2014), “Fluorination of graphene enhances friction due to increased corrugation,”
Nano Letters,
14 (9), pp. 5212-5217.
 Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net.