Potential of van der Waals heterostructures as solid lubricants
Dr. Neil Canter, Contributing Editor | TLT Tech Beat February 2015
A new technique has overcome the problems with lattice matching.
KEY CONCEPTS
•
Heterostructures are substances containing two or more distinct materials.
•
A new process prepares heterostructures by using a scotch-tape method to literally place one material on top of another.
•
Many different heterostructures can be prepared by this technique including the example of one prepared from graphene and tungsten disulfide.
SOLID FILM LUBRICANTS IMPART PROPERTIES such as lubricity, corrosion and chemical resistance to the surfaces of a substrate. These lubricant types are used in extreme operating applications such as aerospace where conventional fluids do not provide adequate performance.
In a previous TLT article, a discussion was provided about the most common solid lubricants used, which are molybdenum disulfide, graphite and polytetrafluoroethylene (
1). The strengths and weaknesses of each of these solid lubricants are detailed.
Molybdenum disulfide generally exhibits the highest load-carrying capability of any solid lubricant but decomposes in an oxidative environment at temperatures above 400 C and is vulnerable to moisture. In contrast, graphite exhibits good high-temperature performance in an oxidative environment and needs moisture to function properly. Molybdenum disulfide can operate in the vacuum of space while graphite cannot.
Suppose there might be a way to develop substances that contain both molybdenum disulfide and graphite. Perhaps such substances might achieve better performance by minimizing the negative aspects seen with each solid lubricant.
These types of substances have been looked at by researchers particularly for use in semiconductors. Hsin-Ying Chiu, assistant professor of physics and astronomy at The University of Kansas in Lawrence, Kansas, says, “Two or more distinct materials have been combined in substances known as heterostructures. An example is combining silicon with germanium as a heterojunction transistor. These two substances do not have the same lattice so they need to interact through a heterojunction.”
The conventional procedure used to prepare heterostructures is known as epitaxial growth. Chiu says, “This requires lattice matching that involves selecting materials with very similar lattices so that electronic and optical properties can be designed for semiconductor applications.”
Lattice matching has limitations because substances with different lattices cannot always be used in heterostructures. A technique has now been developed to overcome the problems with lattice matching and can lead to the development of many different types of heterostructures.
PLAYING WITH LEGOS
Chiu, in collaboration with his associate Hui Zhao, associate professor of physics and astronomy at The University of Kansas, has developed a new process for preparing heterostructures. Chiu says, “We used a scotch-tape method to literally place one material on top of another.”
The heterostructure was prepared from graphene and tungsten disulfide. The process starts by exfoliating a crystal of graphite that had been placed on a sticky polymer such as poly-methyl-methacrylate until only a monolayer (graphene) was identified by spectral analysis. Exfoliation is also used to reduce the size of a tungsten disulfide flake. In both cases, scotch tape is used to remove layers from both materials.
Chiu says, “We then used a micromanipulator to place one material on top of another using a good microscope.” Figure 1 shows an image of the heterostructure with the tungsten disulfide on top and the graphene on the bottom.
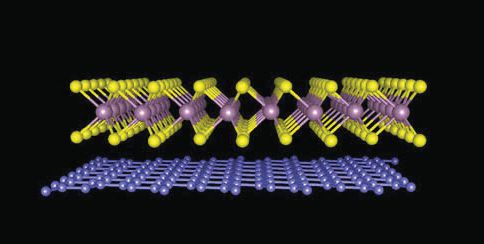 Figure 1. A new process for preparing heterostructures was used to prepare the one shown based on tungsten disulfide on top and graphene on the bottom. Figure courtesy of The University of Kansas.
Figure 1. A new process for preparing heterostructures was used to prepare the one shown based on tungsten disulfide on top and graphene on the bottom. Figure courtesy of The University of Kansas.
The reason this heterostructure holds together is van der Waals forces. Zhao says, “Van der Waals forces are a very broad dipole interaction that enables two neutral substances to become attracted to each other. No charge interactions occur in the example we provided of graphene and tungsten disulfide.”
Chiu says, “The individual arrangement of the atoms in graphene and tungsten disulfide does not change. Atoms in both substances remain strongly bound to their neighbors.”
The initial work was done with graphene and tungsten disulfide because these two materials can be used in a complementary manner to develop new materials for use in solar cells. Zhao says, “Tungsten disulfide is one of the best absorbers of sunlight, and graphene is very effective as a conducting electrode. Hence, this heterostructure is used because the junction can generate sufficient photo-carriers, then quickly be transferred to graphene so that a photovoltage can be generated.”
The researchers evaluated the heterostructure by exciting the tungsten disulfide with a laser pulse and found that almost all of the electrons excited transferred to the graphene in approximately one picosecond.
The success of this approach means that researchers can literally assemble many different heterostructures using substances in a similar fashion to Lego toy bricks. Chiu says, “We envision that this technique can be used to help in the development of multifunctional devices such as solar cells and LEDs. It has great potential for wearable technology.”
The researchers indicate that heterostructures are not restricted to just two substances; many substances can be used to prepare unique combinations. From a solid lubricant perspective, this approach can be used conceivably to assemble heterostructures containing combinations of graphene, molybdenum disulfide and polytetrafluroethylene. It will be interesting to evaluate the lubrication properties of these combinations of heterostructures. Chiu says, “One other application that we feel is possible is the development of new catalysts for chemical reactions.”
Additional information can be found in a recent article (
2) or by contacting Chiu at
chiu@ku.edu or Zhao at
huizhao@ku.edu.
REFERENCES
1.
Gresham, R. (2003), “Solid film lubricants: Unique products for unique lubrication,” TLT,
59 (10), pp. 28-31.
2.
He, J., Kumar, N., Bellus, M., Chiu, H., He, D., Wang, Y. and Zhao, H. (2014), “Electron transfer and coupling in graphene-tungsten disulfide van der Waals heterostructures,”
Nature Communications, DOI: 10.1038/ncomms6622.
 Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net.