High pressure reaction of benzene
Dr. Neil Canter, Contributing Editor | TLT Tech Beat January 2015
The formation of a new solid occurs with benzene reacting in the solid-state.
KEY CONCEPTS
•
A white crystalline powder is formed by subjecting benzene to extreme pressure at room temperature.
•
This solid is composed of three threads of carbon in a structure known as a carbon nanothread.
•
The average thickness of each nanothread is 0.6 nanometers and its formation may be directly related to the diamondoids formed from petroleum oil.
THERE ARE MANY STRATEGIES IN R&D THAT CAN BE TAKEN TO TRY AND CONVERT A SPECIFIC MATERIAL or chemical substance into a desired end material or product. Many times the effort can lead to an unsatisfactory result.
Out of frustration, this can lead to thoughts of literally physically hitting or putting pressure on a specific material or chemical substance to make the process happen in what is believed to be the right way. Such efforts can sometimes lead to success.
For example, in a previous TLT article, researchers found that a solid-like state can be formed by hitting a suspension of cornstarch in water with an aluminum rod (
1). This effect occurs due to the compression of the particles in the suspension, leading to the formation of a rapidly growing solid, which will revert back to a suspension over time once the hitting ends.
Another approach to transforming a specific material into a new product is to literally apply an extreme amount of pressure. John Badding, professor of chemistry at Pennsylvania State University in State College, Pa., says, “High-pressure reactions have been used in organic chemistry for a long time to facilitate reactions. Most of the reactions have been conducted under modest reaction conditions and force reactants into smaller volumes. An example is the conversion of the monomer ethylene into polyethylene.”
One chemical substance that has been looked at in high pressure reactions for over 20 years is the simplest aromatic molecule, benzene. Badding says, “Benzene is far more resistant to reaction under high pressures than other organic molecules because it has a stable six-membered ring structure with all of the carbons in an sp
2 orientation. In previous work, benzene was converted into an amorphous polymer that was not structured and only partially characterized.”
CARBON NANOTHREADS
A new approach has now been taken by Badding and his associates to transform benzene into a more ordered solid. Badding says, “The first thing to consider in the process we developed is that the formation of this new solid occurred with benzene reacting in the solid state. Modest pressure of a few gigapascals converts benzene, which is a liquid under standard conditions, into a solid.”
The researchers then subjected benzene to a pressure that is 20 gigapascals at ambient temperature for one hour and then slowly reduced the pressure at a rate of 2 gigapascals per hour over a 20-hour period. In contrast to past attempts to work with benzene, the resulting product is a white, crystalline powder.
Bright field transmission electron microscopy and x-ray scattering analysis shows that the solid consists of three threads of carbon in what Badding calls a nanothread structure. An image of the carbon nanothread structure is shown in Figure 2.
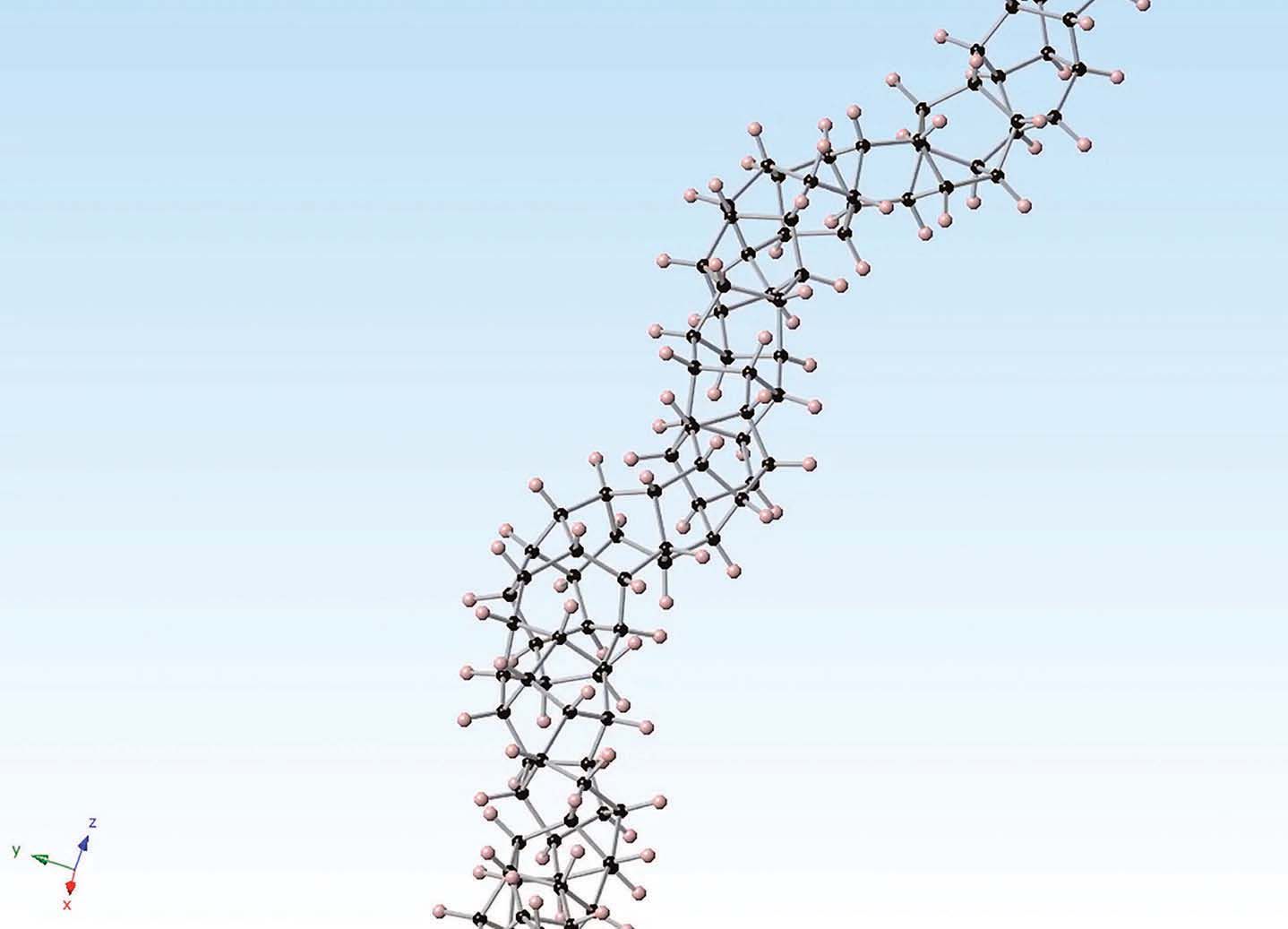 Figure 2. Carbon nanothreads formed by subjecting solid benzene to extreme pressures contain three threads of carbon that have an sp3 configuration. (Courtesy of Pennsylvania State University)
Figure 2. Carbon nanothreads formed by subjecting solid benzene to extreme pressures contain three threads of carbon that have an sp3 configuration. (Courtesy of Pennsylvania State University)
Badding says, “We had a lot of opinions about what to call this unique material. The solid is not a conventional polymer such as polyethylene, which has one strand of carbon atoms. It is also not a ladder polymer that has two strands of carbon atoms. We settled on designating the solid as a nanothread, which is smaller than a nanowire but thicker than a polymer.”
The pressure exerted during the reaction forced the sp
2 configuration of the carbon atoms in benzene into an sp
3 configuration, which displays the structure of a sequence of saturated, cyclohexane rings arranged in a zigzag pattern, similar to the structure of a diamond. Badding says, “One of the reasons we isolated the nanothread may be because the process started with a solid, which contains a series of benzene rings stacked in a column.”
Each nanothread has an average thickness of 0.6 nanometer, and the length of the nanothreads has not been determined. Badding says, “We believe the nanothreads are at least several hundred nanometers long.”
Badding feels that the nanothreads derived from benzene should exhibit exceptional strength and stiffness with light weight. He says, “The structure of the nanothreads will resist bending in all directions. Additional interesting characteristics could be achieved if the nanothreads can be crosslinked.”
Badding says, “The formation of the nanothreads could be directly related to the diamondoids formed from petroleum oil. We believe that diamondoids are derived by subjecting aromatic compounds under pressure in the earth.”
The researchers are now looking to use the same experimental conditions on benzene derivatives such as toluene and xylene. Badding says, “We would like to determine if the high pressure conditions will enable these benzene derivatives to react in the solid-states and produce a material that is ordered.”
Additional information can be found in a recent article (
2) or by contacting Badding at
jbadding@chem.psu.edu.
REFERENCES
1.
Canter, N. (2012), “Impact-activated solidification,” TLT,
68 (10), pp. 12-13.
2.
Fitzgibbons, T., Guthrie, M., Xu, E., Crespi, V., Davidowski, S., Cody, G., Alem, N. and Badding, J. (2014), “Benzene-derived carbon nanothreads with sp
3 bonding,”
Nature Materials, DOI:10.1038/NMAT 4088.
 Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net.