Metalworking corrosion issues and test methods
Jerry P. Byers, CMFS | TLT Webinars October 2014
Evaluating corrosion problems in metalworking and metal-treating applications.
KEY CONCEPTS
•
15 years ago corrosion issues cost the U.S. economy more than a quarter of a trillion dollars.
•
MWFs must control corrosion on the manufactured metal part and the metals comprising the machinery conducting the operation.
•
Factors affecting corrosion include moisture, temperature, air pollution, fluid contaminants and water quality.
MEET THE PRESENTER
This article is based on Webinars originally presented by STLE University. “Metalworking Corrosion Issues & Test Methods” is available at
www.stle.org: $39 to STLE members, $59 for all others.
Jerry Byers is the manager of Cimcool Product Research & Development at Cimcool Fluid Technologies LLC in Cincinnati, Ohio. He holds STLE’s Certified Metalworking Fluids Specialist certification and is a past president of STLE. He’s also a member of the STLE Metalworking Fluids Education and Training Subcommittee, holds a patent for a heavy duty, chlorine-free metalworking fluid, and is the editor of the best-selling book,
Metalworking Fluids, Second Edition, which has been translated into a Chinese edition. You can reach him at
jpbyers@aol.com.
 Jerry Byers
Jerry Byers
CORROSION CONTROL IS ONE OF THE MOST IMPORTANT PROPERTIES OF METALWORKING FLUIDS and metal-treatment fluids used in industry. Fifteen years ago, it was estimated that corrosion problems cost the U.S. economy more than a quarter of a trillion dollars or 3.1 percent of the U.S. gross domestic product at that time.
Metalworking and metal-treatment fluids must control corrosion on both the metal part being manufactured, as well as the metals that comprise the machinery conducting the operation (turning center, grinder, stamping press, washer, etc.).
This article, based on a Webinar presentation sponsored by STLE University, examines the importance of corrosion control in metalworking and metal-treating applications as it relates to five different metals (iron, aluminum, magnesium, copper and titanium) that are commonly used in industry. We’ll take a brief look at important aspects of each metal, which have unique physical and chemical properties, as well as corrosion issues. Common corrosion testing methods also will be presented.
In addition to the composition of the fluids being used, several factors can affect corrosion control, including:
•
Moisture/humidity
•
Temperature
•
Air pollution
•
Fluid contaminants (acids, bases, salts, microorganisms)
•
Water quality in the plant
•
Fluid concentration control
•
Concentration differences across the metal surface
•
Crevices in the surface
•
Contact between dissimilar metals (a galvanic couple)
INDUSTRIAL METALS
Iron. By far iron is the metal with highest usage, due to its relatively low cost and high strength. Small amounts of carbon can greatly increase the strength of iron. Steel has a lower carbon content than cast iron. The main disadvantage of iron is that it corrodes quickly. Even if a manufacturer is machining or forming aluminum parts, the machine tools will be mostly iron and steel. So rust control is a critical property of metalworking and metal-treatment fluids. Within the laboratory, a commonly used method to evaluate rust control is the ASTM D4627 cast-iron chip rust test.
Typical corrosion inhibitors used include amines, amine-borates and amine-carboxylates (amine salts of short-chain, fatty acids). Amines (nitrogen-containing, organic compounds) are critical to obtaining good rust control in water-based fluids. Among the in-plant conditions that can cause rust problems, despite the metalworking fluid, are poor quality water, high microbial counts, high humidity and acidic air contaminants.
 Figure 1. Cast-iron chip corrosion testing in progress.
Aluminum.
Figure 1. Cast-iron chip corrosion testing in progress.
Aluminum. A lightweight metal about a third the density of steel, aluminum is remarkable in its resistance to corrosion. Nonetheless, it does corrode under certain conditions such as attack by salts (for example, chlorides), high pH and when in contact with other metals. A simple way to evaluate the potential for a fluid to stain aluminum is to place a clean strip of the metal alloy in a beaker of fluid so that it is about half immersed and leave it in the fluid overnight.
 Figure 2. Example of bimetallic or galvanic corrosion. A round steel specimen (shown on the left) and cast-iron specimen (shown on the right) were placed on an aluminum strip wetted with metalworking fluid.
Figure 2. Example of bimetallic or galvanic corrosion. A round steel specimen (shown on the left) and cast-iron specimen (shown on the right) were placed on an aluminum strip wetted with metalworking fluid.
A more complex procedure is ASTM F1110 Standard Test Method for Sandwich Corrosion Test or commonly referred to as the Boeing sandwich test. This involves putting a piece of filter paper wetted with the fluid in question between two aluminum panels and placing the sandwich at elevated temperatures and cycled humidity for seven days, as shown in Figure 3. Any stain worse than that caused by deionized water is a failure. The fluid must pass both in concentrate form and at the recommended dilution.
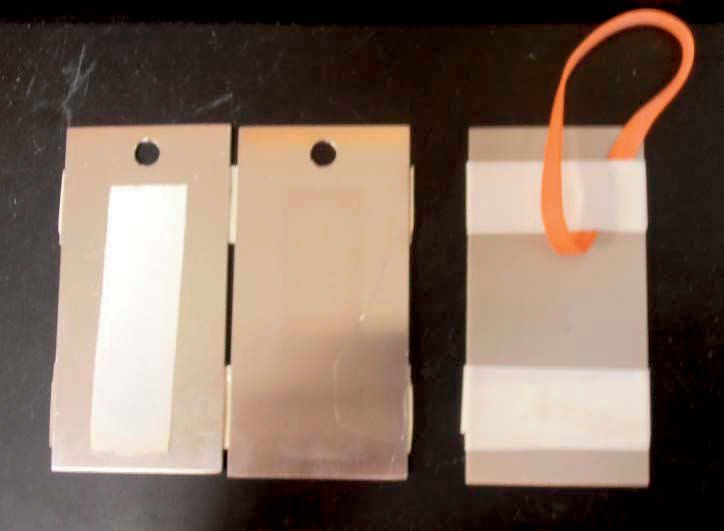 Figure 3. Two aluminum panels with wetted filter paper (shown on the left) and the completed sandwich taped together and ready to hang in humidity cabinet (shown on the right).
Figure 3. Two aluminum panels with wetted filter paper (shown on the left) and the completed sandwich taped together and ready to hang in humidity cabinet (shown on the right).
A third corrosion mechanism often observed with aluminum is bimetallic corrosion. This occurs when an aluminum workpiece is clamped onto a ferrous metal table and held in place with steel or cast-iron clamps. Corrosion will be observed on the aluminum at the points of contact with the iron alloys. Fluids are sometimes evaluated for their ability to prevent bimetallic corrosion.
Commonly used corrosion inhibitors for aluminum include silicates and phosphate esters.
Magnesium. This is the third most commonly used structural metal, following steel and aluminum. Its density is two-thirds that of aluminum, but it has good strength. Its two main drawbacks, however, are that it will burn if it gets hot enough, and it reacts with water to generate hydrogen gas, which is explosive. The latter property is particularly problematic if a water-based fluid is being considered. One test method is to place magnesium chips in the fluid and then collect and measure the volume of hydrogen gas generated during a set period of time. Metalworking fluid products can be ranked by the volume of hydrogen produced, as well as by degree of stain on the magnesium surface.
Copper. This is the most versatile and durable of all metals. Copper is malleable, ductile and long lasting, as well as a better conductor of heat and electricity than any other metal except silver. Copper is commonly found in coins, motors, computers, refrigerators, air conditioners, microwaves, automobiles, electrical wiring, water pipes and appliances. However, when it is used in air conditioners, it is susceptible to a mode of failure called formicary corrosion or “ants nest” corrosion. This starts as a tiny pit on the surface that then branches out beneath the surface into a series of microscopic tunnels reminiscent of the tunnels in an ant farm.
Some experts say formicary corrosion is caused by hydrolytic breakdown of drawing lubricants to form low levels of formic acid and acetic acid. One test method for copper-forming fluids is to combine 90 grams of lubricant with 10 grams of water and 1 gram of alumina and then reflux for 48 hours. The mixture is then extracted with 90 grams more water. A passing result is less than 20-ppm formate or acetate. An alternate procedure is ASTM D2619.
Titanium. This metal has the highest strength-to-weight ratio of any metal and thus is being used increasingly to reduce weight and improve fuel economy in aerospace, military and industrial applications. It is also used for medical and dental implants due to its biocompatibility.
Titanium also has a low modulus of elasticity compared to steel, making it more springy than steel. This means that the workpiece tends to move away from the cutting tool unless heavy cuts are maintained. Titanium is a poor conductor of heat, so heat does not dissipate quickly. Therefore, during cutting operations the heat of deformation is concentrated on the cutting edge of the tool and on the tool face.
Although titanium is relatively corrosion-resistant, it is susceptible to stress-corrosion cracking at elevated temperatures—a particularly important failure mode for aerospace applications. ASTM F945 is a procedure for evaluating stress-corrosion cracking. Prestressed titanium specimens are exposed to the test solution for one minute, allowed to dry, and then baked under one of two conditions. Method A is at 900 F for 8 hours, while Method B is at 500 F for 168 hours. A 100-ppm sodium chloride solution serves as the positive control.
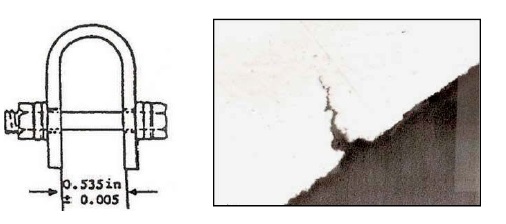 Figure 4. (Left) Titanium bars are stressed by bending into a U-shape. (Right) Microphotograph of stress crack in titanium bar following ASTM F945.
HOT CHIPS
Figure 4. (Left) Titanium bars are stressed by bending into a U-shape. (Right) Microphotograph of stress crack in titanium bar following ASTM F945.
HOT CHIPS
Over the years, manufacturing industries have had to deal with a strange problem described as hot chips. This is a situation where a bin of metal chips (for example, the sawdust from a metal-cutting or grinding operation) suddenly starts generating heat and smoke. Under some conditions, it can actually burst into flame. In at least one instance, a rail car full of metal grinding swarf began burning in a Chicago rail yard. Such an incident can quickly become a concern for governmental agencies such as the EPA and put manufacturing companies in a very negative light.
Earlier we talked about corrosion issues involving five common industrial metals and some of the corrosion test methods used for each. Hot chips are a related issue. How is it possible that metal particles can start smoldering on their own and even burn like a trash fire? If you answered corrosion, you’re absolutely correct!
If you have ever been hunting or at a football game in the winter, you might have purchased little packets, about 2 inches-by-3 inches in size, that you activate by pulling a tab off and then putting them into your pockets or gloves. The packet gradually gets warm and can keep you toasty for several hours. These hand-warming packs contain little more than cast-iron powder, mixed with carbon powder, some water and, perhaps, a little salt to accelerate the corrosion process. The reaction that generates the heat starts when the tab is pulled, exposing the mixture to oxygen from the air. The oxygen and water slowly oxidizes (rusts) the cast iron, generating a considerable amount of heat. This is a quite useful reaction and easily controlled in little packets, but a large hopper full of tiny metal pieces in warm humid weather is a different matter. Any oily residue remaining on those metal chips will help to generate smoke and flames.
We studied this reaction in the laboratory by placing 20 grams of iron powder in a small insulated beaker with varying amounts of tap water, plus a thermocouple to measure the temperature. We found the following:
1.
Chips (cast iron) could go from 64 F to 190 F within 12 minutes, as shown in Figure 5.
2.
Cast iron was the metal most likely to generate heat—much more than steel fines. A mixture of aluminum and cast-iron chips seemed to be no worse than cast iron alone (
see Figure 5).
3.
The amount of heat generated is affected by the moisture content. The maximum amount of heat is generated when there is about 10-15 percent water by weight (
see Figure 6).
4.
Fretting corrosion exacerbates the amount of heat generated. (Fretting corrosion is caused by vibrations, as it rubs off the oxide layer to expose a fresh surface ready to rust. Thus, the problem becomes even bigger when the chips were transported by rail car or truck.)
5.
The most effective way of preventing this heat generation is to:
a.
Shut off the air supply, if possible
b.
Spray the chips with a corrosion inhibitor.
6.
When a dilute solution (about 0.5 percent) of an oil-free corrosion inhibitor was applied, the temperature rise reached a maximum of only 84 F (barely warm). See Figure 7. Some inhibitors were more effective than others. A pump-up type garden sprayer would work as the applicator, but it is important to get the rust inhibitor to penetrate down deep into pile of chips—not just on the top surface.
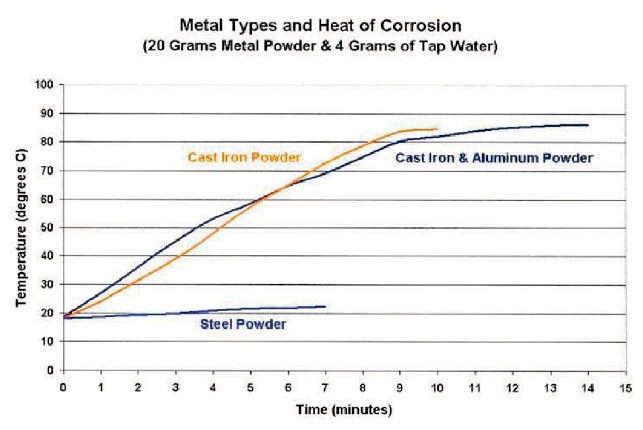 Figure 5. Comparison of the heat generated from different powdered metals.
Figure 5. Comparison of the heat generated from different powdered metals.
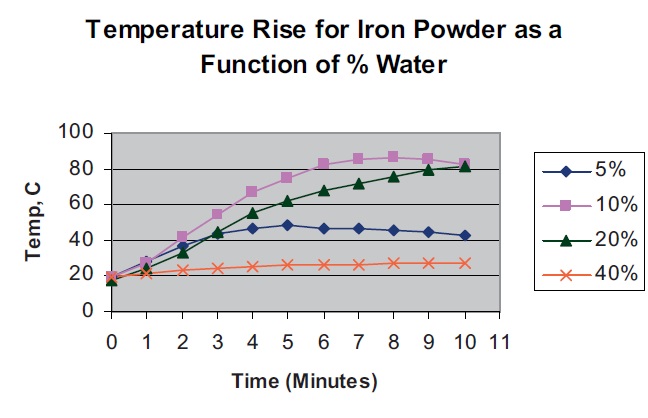 Figure 6. Graph of the temperature rise for cast-iron powder with varying amounts of water.
Figure 6. Graph of the temperature rise for cast-iron powder with varying amounts of water.
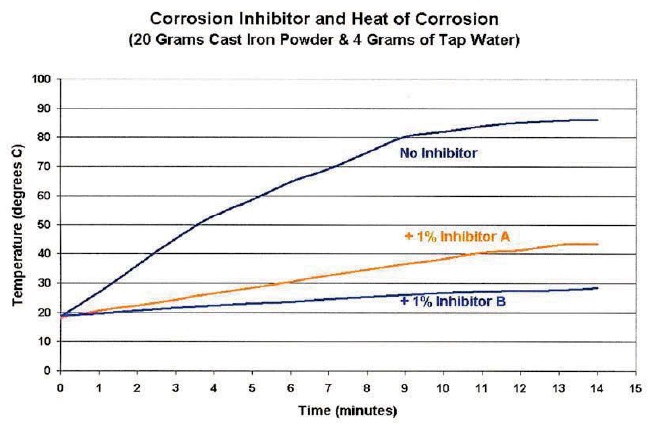 Figure 7. This graph demonstrates the beneficial effect of adding two different rust inhibitors.
Figure 7. This graph demonstrates the beneficial effect of adding two different rust inhibitors.
An obvious question is why doesn’t the metalworking fluid residue on the metal chips prevent oxidation of the chips in the hopper and thus prevent spontaneous heating? The answer is that it does to a certain degree, but the corrosion inhibitor from the metalworking fluid is eventually depleted by trying to protect the vast surface area represented by the metal fines. This requires additional reinforcement in the form of added rust preventive.