Aluminum Metalworking Fluids
Jeanna Van Rensselar, Contributing Editor | TLT Feature Article August 2014
Between cans and cars, the markets for aluminum and its metalworking fluids are exploding.
KEY CONCEPTS
•
Lightweight, durable, attractive and infinitely recyclable, aluminum use is taking off.
•
Aluminum metalworking fluids are a highly specialized niche that is expanding at the same pace.
•
Because aesthetics are paramount in many aluminum applications, these fluids need to do everything that other metalworking fluids must do without staining the metal.
Formulating aluminum metalworking fluids is no easy task. Perkins Products’ technical director Lon Fanning calls it a juggling act. Lubrizol’s technical fellow and Certified Metalworking Fluids Specialist™ Tom Oleksiak calls it a challenge.
“Formulating high-quality aluminum machining coolants is particularly difficult because you are balancing biostability, or bioresistance, with the propensity of these fluids to stain aluminum,” Fanning explains. “Most of the ingredients used to maintain pH and alkalinity also will stain. So the question then becomes, do you sacrifice alkalinity for the sake of corrosion control? Do you use a stain inhibitor, which can have its own negative consequences? Plus you need lubricity that is activated at relatively low temperatures. These additives also can become bug food. Of course, our customers want to use one fluid for everything, including stainless, and most end-users perform some sort of recycling, which has its own unique set of challenges.”
Considering the current explosion in worldwide aluminum use, developing aluminum metalworking fluids is a task that promises ample reward.
WHAT YOU DON’T KNOW ABOUT ALUMINUM
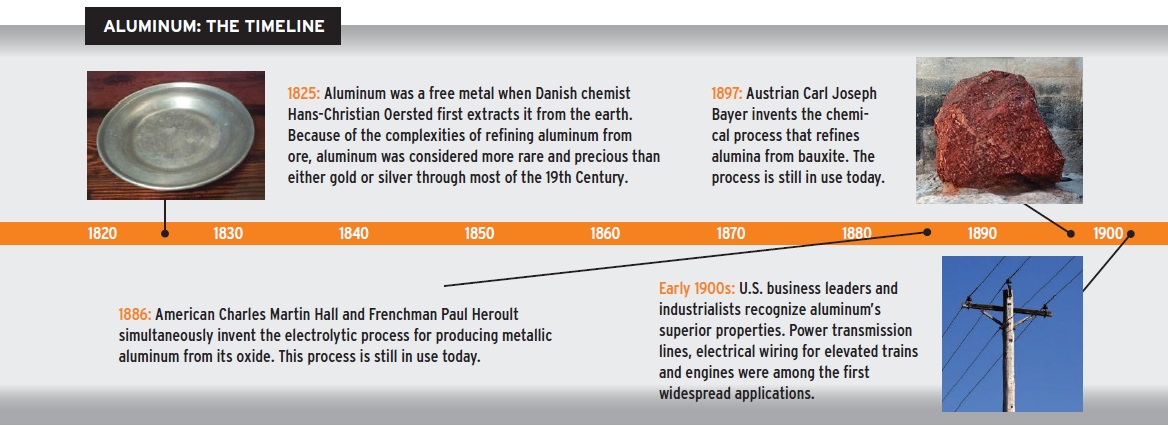
Because of the ordeal of refining aluminum from ore, aluminum was considered more precious than gold or silver through most of the 19th Century. In the mid-1800s, Napoleon III, the first president of the French Republic, served state dinners on aluminum plates. Lesser guests were served on cheaper gold or silver plates. In fact, because more than 75 percent of the aluminum ever produced is still in use today, the foil you just used to wrap leftover brats could be descended from one of Napoleon III’s plates.
Like other non-ferrous metals, aluminum has a big strength-to-weight advantage over ferrous metals. Aluminum is naturally corrosion resistant and non-magnetic, which makes it the material of choice for electronics and wiring.
Nearly all aluminum was originally extracted from bauxite, a mixture of hydrous aluminum oxides, aluminum hydroxides, clay minerals and insoluble materials such as quartz, hematite, magnetite, siderite and goethite. The aluminum minerals in bauxite can include gibbsite Al(OH)
3, boehmite AlO(OH) and diaspore, AlO(OH) (
1). Bauxite is technically not a mineral but a rock.
The leading bauxite-producing countries include Australia, China, Brazil, India, Guinea, Jamaica and Russia. The U.S. has very large deposits of bauxite in Arkansas, Alabama and Georgia. But little of U.S. bauxite is mined since aluminum processing uses a significant amount of electricity and tends to be processed where electricity costs are very low. Right now there are more than 4,100 aluminum production facilities in operation worldwide and more than 155,000 aluminum industry workers.
Aluminum is the most abundant, naturally occurring metal in the earth’s crust. It is lightweight, durable and recyclable, and it’s one of the more attractive low-cost metals—making it ideal for consumer goods like tablets and smartphones. In fact, consumer electronics is a rising new market.
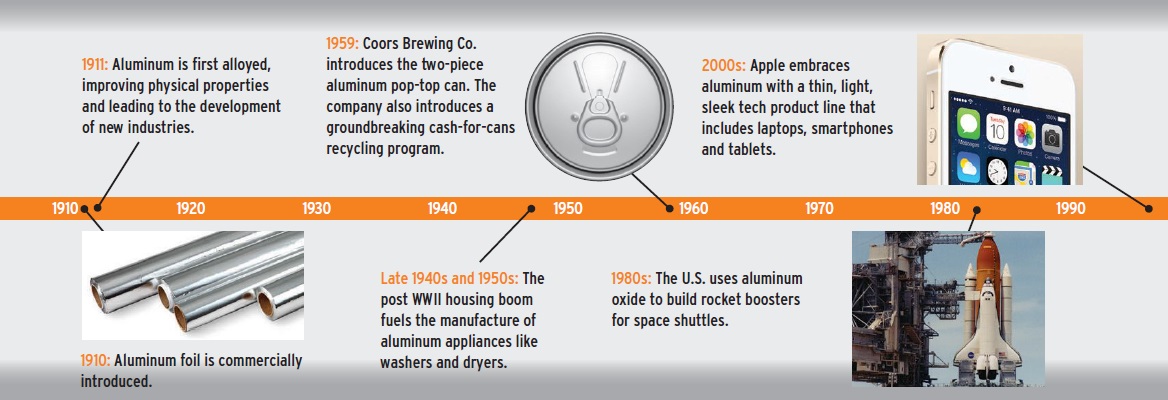
Aluminum is almost always alloyed to improve properties such as strength. Most common alloys are copper, zinc, magnesium, manganese and silicon. For example, aluminum foil and drink cans contain 1-8 percent alloy.
For heavy industrial markets, aluminum is a durable, strong, corrosion-resistant material that is also lightweight and easily formed—all key attributes for heavy industry applications.
Because aluminum retains its properties indefinitely, it is infinitely recyclable and the most eco-friendly metal. The process of recycling aluminum reduces the amount of energy required for aluminum production by more than 90 percent over primary production.
It is the only commonly recycled material that more than pays for the cost of its own collection. This is pretty remarkable considering that the aluminum industry pays out more than $800 million annually for empty aluminum cans.
But even without recycling, bauxite reserves will last for centuries. Matt Meenan, director of communications for the Aluminum Association in Arlington, Va., says, “We expect that there will be an abundant supply of aluminum for many generations to come.”
While beverage cans account for the biggest share of the aluminum market, auto parts are on the rise. “One exciting area the industry is watching is the automotive sector where many carmakers are starting to look at using high-strength, military-grade aluminum to reduce weight and increase fuel efficiency,” Meenan says. “Most notably, Ford recently announced that the 2015 Ford F-150—the best-selling car in the U.S.—will shave 700 pounds off its total weight by moving to an all-aluminum body. We’ve seen growth in aluminum auto sheet shipments of around 15-20 percent per year over the last several years.”
 ALUMINUM PROCESSING
ALUMINUM PROCESSING
There are two ways of producing aluminum:
1.
Turning raw bauxite into aluminum, mining bauxite deposits from the earth and processing them electro-metallurgically.
2.
Recycling—turning aluminum scrap into marketable aluminum.
 Aluminum cans in preparation for recycling.
Aluminum cans in preparation for recycling.
From there, different processing methods and alloys are employed to turn aluminum into required densities, shapes and strengths. These methods include:
•
Casting (usually in a sand mold) is a straightforward, inexpensive and versatile way of forming aluminum. It is a common way of producing high-volume parts (such as auto parts) that require little or no machining.
•
Sheet rolling involves passing aluminum between pressurized rolls that produce long, flat pieces of aluminum plate, sheet and foil. Sheet is the most widely used form of industrial aluminum. It’s found in airplane and automotive bodies, food and beverage cans and building facades.
•
Extrusion is used to form longer, thinner pieces of aluminum such as rod, bar and wire. Because of its low cost and flexible tooling, the extrusion process facilitates fast product development— manufacturers can speed prototype and testing phases by quickly creating and evaluating designs. Rivets, nails, screws and bolts are produced this way, as are consumer products such as zippers and twist ties.
•
Forging is a process that involves pressing or pounding the metal under great pressure to produce high-strength parts. There are three primary types: open-die forging, closed-die forging and ring-rolled forging. Forged aluminum is ideal for applications such as sports cars and racecars where performance, safety, speed and energy efficiency are important.
•
Pigments and powder are produced by melting aluminum ingot in a gas furnace and spraying the molten metal into a fine powder. Aluminum pigments and powder are found in products ranging from lotions and cosmetics to lightweight concrete.
 Bauxite with a penny for comparison. (Courtesy of the U.S. Geological Survey and the Mineral Information Institute)
Bauxite with a penny for comparison. (Courtesy of the U.S. Geological Survey and the Mineral Information Institute)
ALUMINUM FUN FACTS (2)
•
75 percent of all aluminum ever produced is still in use today. Aluminum is 100 percent recyclable and retains its properties indefinitely.
•
Pound for pound, aluminum absorbs twice the auto collision energy of steel and performs as well in an accident. Aluminum crash rails fold up like an accordion, which dissipates and directs energy away from the vehicle’s occupants. Aluminum also provides advantages in stopping distance, handling and performance.
•
Aluminum foil provides a complete barrier to light, oxygen, moisture and bacteria. For this reason, foil is used extensively in food and pharmaceutical packaging. Aluminum foil is also used to make aseptic packaging, which allows storage of perishable goods without refrigeration.
•
Aluminum is superior to steel and iron in its ability to reflect the infrared (heat) rays of the sun. Roofs made from aluminum reflect up to 95 percent of the solar energy that strikes them, dramatically improving energy efficiency.
•
Unlike wire conduit made from steel, rigid aluminum does not spark, resists corrosion and doesn’t rust. These properties of aluminum are vitally important for electrical applications within coal mines, grain elevators and refineries.
•
Once an aluminum can is dropped off for recycling, the recycled version can be back on the shelf in as little as 60 days.
•
Recycling aluminum saves more than 90 percent of the energy that would be needed to create a comparable amount of the metal from raw materials.

ALUMINUM MWF REQUIREMENTS
As a rule, aluminum is not a demanding metal to machine. In fact, some aluminum alloys can be machined without any lubricant or cutting fluid. Given this, logic would dictate that it’s OK to use a general purpose fluid (and many people do), but that’s where problems arise.
“There are no one-size-fits-all additives for aluminum,” Oleksiak explains. “Aluminum can be machined or formed in a wide variety of alloy types. These alloys have different machining lubrication demands and also may be more or less sensitive to aluminum stain. Therefore, development of an aluminum machining fluid must be a careful blend of well-chosen additives that meets the customer’s expectations. A recent trend in the industry is the use of a high-oil semisynthetic of greater than 50 percent oil that meets many aluminum application needs.”
So even though aluminum is relatively easy to machine, it does require specialized fluid that provides the following:
Staining/corrosion prevention. In the case of aluminum, stains are not the same as corrosion. Staining is defined as a surface defect that does not affect structural integrity. But staining is still highly undesirable for two reasons:
1.
It can interfere with electrical contacts.
2.
It compromises aesthetics—important since aluminum is the shell material of choice for some of the world’s most expensive electronics.
Stains are usually pale yellow or slightly golden. They can develop when synthetic fluids approved for use with aluminum are left in the system so long that the inhibitors become depleted and alkalinity rises. In fact, the most common cause of aluminum staining with metalworking fluids is high pH, which dissolves the naturally protective oxide layer and exposes the piece to corrosion. Stains can occur even without the presence of water—making formulating non-staining lubricants even more difficult.
Oleksiak explains that metalworking coolants often have a pH above 9 to protect ferrous metals from staining and corrosion. The high pH of these synthetics can retard corrosion on ferrous metals but actually
lead to corrosion on non-ferrous metals (
3). At a pH below 9, aluminum forms a protective oxide that prevents corrosion. At a pH of 9 and greater, this protective oxide changes in chemistry and becomes dark in color.
“These dark stains are usually unacceptable in the final machined product,” Oleksiak says. “Fortunately, additives can prevent these stains. Most of the additives that are effective at preventing aluminum stains contain phosphorus.”
The natural corrosion resistance of aluminum is a minor miracle. When exposed to air, an oxide forms seamlessly on the surface. Even if the oxide is scratched, it will quickly repair itself. The electrochemical process of anodizing produces an even thicker layer of protection. If the anodizing bath is refrigerated during the process, it will yield an even thicker, ceramic-like oxide coating.
On the other hand, aluminum is very vulnerable to attack by aqueous acids and alkalis. As Oleksiak mentioned, this is a big issue with water-based coolants used in aluminum machining. This corrosion ranges from gray to black and is the result of high alkalinity.
Good lubrication. Oleksiak points out that challenges with aluminum lubrication are related to lubricity additive chemistry, as well as wetting of the coolant on the aluminum surface. With steel, there are numerous extreme pressure (EP) additives available, including chlorine, phosphorus and sulfur. Almost all EP additives demonstrate activity on steel. These additives chemically bond to the iron surface and prevent metal-to-metal contact.
“But finding additives that work on aluminum when traditional boundary additives—for example, fatty acids— fail is a much bigger challenge,” Oleksiak says. “Chlorine, phosphorus and sulfur additives may help, but with aluminum it is much more chemistry specific. In other words, not all phosphate esters that improve lubricity on steel will improve lubricity on aluminum.”
Oleksiak adds, “Coolant wetting also is more difficult on an aluminum surface versus a steel surface. The surface energy of an aluminum oxide surface is significantly lower than steel, and this property makes coolant wetting more of a challenge. Often, wetting agents are added specifically to address wetting on an aluminum surface. This means it’s important to evaluate the wetting properties of a coolant in an aluminum operation.”
THE ALL-ALUMINUM FORD F-150 (4)
Ford introduced the all-aluminum F-150 pickup at the North American Auto Show in Detroit last January—weighing 700 fewer pounds than its predecessor. According to auto industry expert Michael Wayland, Ford engineers have been working on the pickup since 2008. The vehicle is now the lightest truck in the half-ton class and more fuel-efficient than any Ford truck ever produced. Interestingly, this isn’t Ford’s first foray into an all-aluminum truck—it produced another in 1930.
The F-150 represents a trend (driven mostly by increasingly stringent fuel efficiency regulations) toward including more weight-saving measures such as aluminum components in vehicles. And better fuel economy isn’t the only benefit; this pickup can tow and haul more and handle, brake and accelerate better than its weightier counterparts.
Wayland calls the vehicle “arguably one of the most important vehicles in decades.” The truck is produced in Michigan and Missouri and goes on sale this fall (
5). Although Ford has not revealed the price, Edmunds, the used-car pricing guide, is not projecting a significant price increase over the 2014 F-150 (
6). But because aluminum is more expensive to repair than steel, the price of repairs and insurance could be higher.
 Ford unveils the all-aluminum F-150. By utilizing an all-new high-strength, military-grade, aluminum alloy body, the truck will weigh 700 lbs. less, thereby improving handling and improving efficiency. (Courtesy of Ford Motor Co.)
Ford unveils the all-aluminum F-150. By utilizing an all-new high-strength, military-grade, aluminum alloy body, the truck will weigh 700 lbs. less, thereby improving handling and improving efficiency. (Courtesy of Ford Motor Co.)
Soap formation prevention. Soap formation is another problem with aluminum coolants. Aluminum reacts with fatty acids in an aqueous environment to form aluminum fatty acids soaps. These soaps tend to be more viscous and more tenacious on surfaces than other metal soaps.
“For this reason, the level of fatty acids in aluminum coolants should be minimized if possible,” Oleksiak says. “An alternative way to address this issue is to add a surfactant chemistry that wets and disperses the soaps.”
One of the current issues with aluminum metalworking fluids is whether fluids used in high-speed cutting operations need to be of a quality that is as high as low-speed counterparts—bearing in mind that high cutting speeds are associated with lower machining forces, a better surface finish and reduced tool wear. To arrive at an answer, Quaker Chemical Corp. and The Pennsylvania State University conducted a study which concluded that the use and selection of high-speed metalworking fluid can impact machining performance and potentially yield further improvements in the quality of the part produced, as well as the tool life (
7).
“It’s funny, I have been doing this for over 20 years now. Improvements have been made, but these issues haven’t changed,” says Andy Johnson, director of technology for Wallover Oil in Strongsville, Ohio.
Tool protection. A good metalworking fluid promotes tool life by reducing or eliminating the temperature spikes of the work piece, allowing for faster cutting and less friction. It also keeps the working edge lubricated, reducing wear on the cutting tip and preventing friction. Lack of lubricity can lead to
build-up edge where aluminum fines stick to the tool and negatively influence the metalworking process.
A third way lubricants protect tools is by preventing rust on machine components. “In general, we formulate toward the machining process with the shortest tool life,” Johnson says. “This is often, but not always, tapping.”
ALUMINUM IN THE AUTO INDUSTRY (8)
•
Ducker Worldwide, a leading consulting and research firm, estimates that the average amount of finished aluminum per vehicle was 343 pounds in 2012, up from 327 pounds in 2009.
•
Aluminum is expected to double its 2008 share of the average light vehicle material mix to 16 percent by 2025.
•
Aluminum is currently the dominant material for powertrains, heat exchangers and road wheels, and it is rapidly gaining market share for hoods, trunk lids, bumpers, steering knuckles and suspension arms.
•
Projected 2025 average vehicle weight (not necessarily size) will be reduced by 408 pounds or 10 percent from 2008 levels.
•
Aluminum is gaining share, while both traditional and high-strength steels are declining.
•
Advanced high-strength steels (AHSS) are also growing at the expense of inferior steels. But aluminum replaces more than twice as much inferior steel as AHSS.
•
Consumers will see high-volume, aluminum-bodied vehicles on the road before 2020.
FORMULATION CONSIDERATIONS
Oleksiak says that in addition to the standard formulation considerations for metalworking fluids, when developing a coolant for aluminum it’s also important to understand the type of operations used in the machining process, the types of alloys machined and system parameters such as sump size and recirculating time for the emulsion system.
“Aluminum machining technology is following other industries in that we are seeing smaller sumps and greater pressures in thru-tool cooling, chip blasters, super flush, etc.,” Fanning says. “Because of this, foam control is imperative. Foam makes a very poor lubricant. But defoamers are just Band-Aids. You must start with low-foaming ingredients to achieve a low-foaming coolant.”
Johnson adds that working with recycled aluminum creates additional issues.
“We are observing more residues with recycled cast aluminum alloys, but the casting process can introduce more variables than the liquid metallurgy. However, we can still test our formulations for stain inhibition and machinability accurately on these metals.”
Fanning says that he is seeing the use of aluminum and its alloys steadily increasing. “The combination of lightweight and the strength of alloys is unbeatable, particularly in automotive applications,” he explains. “We feel that soluble oils and high-oil-content semisynthetic metalworking fluids will continue to dominate the market. You need the wetting and hydrodynamic lubrication provided by these products.”
But Fanning and Oleksiak both say the metalworking industry as a whole continues to be impacted by the effects of REACH and GHS regulations—especially where essential raw materials and biocides are being removed from the market (
4).
“Everyone knows about Buckman pulling out of the triazine biocide market and Dow withdrawing their oxazolidines, but what you don’t hear about are the specialty amides and other surfactants that are no longer available,” Fanning says. “Regulation is pushing us toward very vanilla, generic formulations. In the end, it’s the customer who pays the price.”
From his end, Meenan is optimistic. “We continue to be encouraged by how the industry is positioned in North America,” he says. “The industry shipped more than 24 billion pounds of aluminum last year for the first time since 2007, and demand is up around 30 percent in the past four years alone. Use of aluminum in the U.S. and Canada is now approaching the record-setting levels set in the mid- 2000s. As brands from Ford to Apple to the U.S. military are discovering, aluminum has the greatest potential of perhaps any material on the market to drive energy-saving innovation in modern manufacturing.”
REFERENCES
1.
From:
here.
2.
From:
here.
3.
A pH of 7 is neutral; 0-7 is acidic; and 7-14 is alkaline. A high pH, greater than 9, will protect ferrous metals but will adversely affect the corrosion control of non-ferrous metals such as aluminum, brass and bronze.
4.
Actually, with a 95 percent aluminum body, it is only mostly aluminum.
5.
From:
here.
6.
From:
here.
7.
From:
Metalworking Fluid Performance in Aluminum High Speed Machining, click
here.
8.
From Ducker Worldwide Report for The Aluminum Association: Aluminum in 2012 North American Light Vehicles, click
here.
9.
REACH, (the European Union’s Registration, Evaluation, Authorization and Restriction of Chemicals program) and GHS (the United Nations’ supported Globally Harmonized System) are two separate regulatory programs that facilitate and promote protection from chemical hazards.
 Jeanna Van Rensselar heads her own communication/ public relations firm, Smart PR Communications, in Naperville, Ill. You can reach her at jeanna@smartprcommunications.com
Jeanna Van Rensselar heads her own communication/ public relations firm, Smart PR Communications, in Naperville, Ill. You can reach her at jeanna@smartprcommunications.com.