Functionalizing hydrocarbons derived from natural gas
Dr. Neil Canter, Contributing Editor | TLT Tech Beat June 2014
Researchers develop an approach to functionalizing hydrocarbons through a reaction that produces alcohols at lower temperatures.
KEY CONCEPTS
•
Current approaches to functionalizing hydrocarbons from readily available natural gas are energy intensive and expensive.
•
A new approach to functionalizing hydrocarbons known as the C-H activation reaction produces alcohols at much lower temperatures through a more efficient process.
•
In contrast to current approaches, the new process can efficiently functionalize the three main hydrocarbons (methane, ethane and propane) present in natural gas.
THE GROWTH IN PRODUCTION OF NATURAL GAS in the U.S. through the use of techniques such as hydraulic fracturing has been significant. Of particular note is the dramatic improvement in the use of a technique known as horizontal drilling. This has led to major increase in natural gas production in such locations as the Marcellus Shale, which has seen natural gas output increase by six million cubic feet per day over the past seven years. This growth means that natural gas production in the U.S. is estimated to increase by 56 percent through 2040 (
1, 2).
As a result, there is an increased incentive to evaluate natural gas as a fuel. There are existing commercial technologies for conversion of natural gas to fuels, including the Fischer- Tropsch (FT) process for conversion of natural gas to diesel and the MTG processes based on the conversion of methane to gasoline via methanol.
Those working in the lubricant industry also know the first approach as the gas-to-liquid pathway used to prepare the newest type of base oils. Roy Periana, professor of chemistry at The Scripps Research Institute in Jupiter, Fla., characterizes the Fischer-Tropsch approach as extremely expensive. He says, “There is a huge CAPEX that can represent approximately 60-70 percent of the overall cost of the fuels. It also is massively energy intensive and has a large physical footprint.”
The fuels generated from these processes are more expensive than from petroleum. Periana notes that while these technologies are being utilized and new plants are being considered, they will likely not be widely deployed.
The high CAPEX and large footprint are all a direct result of the use of high-temperature, energy intensive chemistry, e.g., reforming for the conversion of natural gas to syngas (a mixture of carbon monoxide and hydrogen gases) that is required in the FT and MTG processes. This leads to expensive reactors, separations and heat management.
To address this limitation, there is research underway to develop lower temperature processes, 200-300 C, for the conversion of natural gas to chemicals and fuels that can lead to dramatic reductions in the CAPEX, energy requirements and the size of the physical footprint.
Periana says, “There is no inherent requirement for the use of high-temperature processes to convert natural gas to fuels and chemicals. It is simply the lack of efficient chemistry for the conversion of CH bonds in the alkanes, methane, ethane and propane that make up the bulk of natural gas.”
Periana indicates that alkanes can be very resistant to reaction with current chemistry because these CH bonds are some of the strongest known in chemistry.
Periana says, “Theoretically, one should simply be able to mix natural gas with air and utilize the desired lower temperature conditions to directly generate useful chemicals such as alcohols or olefins that can be converted to fuels or other materials. The problem is existing, solid metal oxide- based, oxidation catalysts that can operate at lower temperatures (200-400 C) primarily generate carbon dioxide in a radical reaction that is very unselective as potentially desirable products such as alcohols or even olefins preferentially react to generate carbon dioxide.”
Periana indicates that current FT or MTG syngas-based technologies get around the selectivity issue by utilizing high temperature to rip the bonds apart. He adds, “The laws of thermodynamics minimize carbon dioxide formation, but the price paid is a high CAPEX, inefficiency and a large physical footprint.”
C-H ACTIVATION REACTION
Periana and his research associates have now demonstrated that alkanes can be functionalized directly to generate alcohol products with high selectivity at a much lower temperature (150-250 C) than commercial processes by using a reaction known as C-H activation. “The reaction utilizes various metal cations designated as MX. The beauty of the reaction is that radicals are not involved and reactions can be very rapid and selective,” he says.
“The reactions can be carried out at lower temperatures because when the CH bond is being broken, a new bond is being simultaneously formed to the metal centers to generate metal alkyl intermediates, M-C. No radicals are involved and the chemistry of the alkyl fragment, C, is always controlled by the metal center, M,” says Periana.
In a subsequent reaction, these M-C intermediates generate the desired product, C-X.
Previous attempts to accomplish this process required super acid media that lead to expensive separations, utilized expensive precious metals such as platinum and were not selective with ethane or propane that is present in natural gas. Periana says, “The exciting chemistry we now discovered is that we can use inexpensive main group metals salts such as thallium or lead to break the CH bonds of alkanes by the C-H activation reaction.” Equally important, the reactions can be carried out in non-super acids such as acetic or trifluroacetic acid to generate the corresponding alcohol esters.
Figure 3 shows the chemistry for reacting methane with thallium (III) trifluroacetate to generate a methyl thallium intermediate that leads to the methanol ester. During the reaction, the thallium species is reduced to thallium (I) monofluoroacetate. By addition of water, the ester can be hydrolyzed to form the alcohol, methanol.
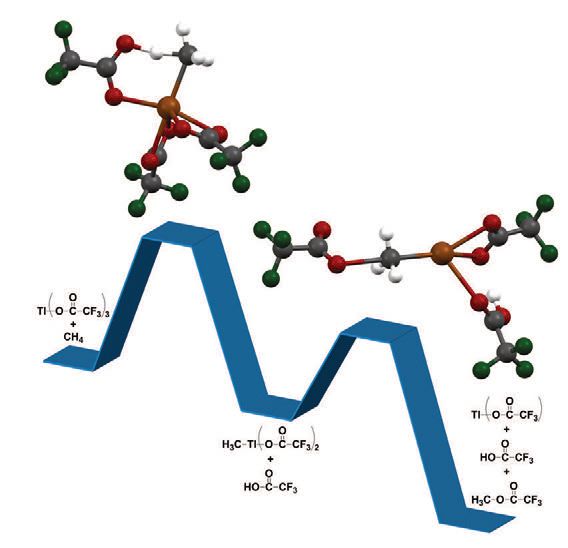 Figure 3. A new selective process for functionalizing hydrocarbons shows how the reaction of methane with a thallium (III) trifluoroacetate proceeds through a methyl thallium intermediate. (Courtesy of The Scripps Research Institute)
Figure 3. A new selective process for functionalizing hydrocarbons shows how the reaction of methane with a thallium (III) trifluoroacetate proceeds through a methyl thallium intermediate. (Courtesy of The Scripps Research Institute)
Periana says, “A real cool aspect of this chemistry is that the reactions are efficient, not just for methane but also for ethane and propane, which are also present in natural gas.” Methane, ethane and propane, separately or as a mixture, will react with these main group salts. This feature could allow natural gas to be used for the generation of chemicals without the need for cryogenic separation utilized in current commercial processes.
The reactivity of these hydrocarbons is different with propane more reactive than ethane, which is more reactive than methanol. Periana says, “It may be possible to adjust the kinetics chemically so that propane can be reacted preferentially.”
Acid media such as acetic, trifluoroacetic acid or other non-super acids are used to facilitate the reactions of the metal salts, as well as protect the alcohol products from further reacting by protonation and/or formation of the esters. Periana says, “We found that the alcohol esters are at least one thousand times less reactive than the corresponding alkane.”
This work shows that alkanes can now be functionalized to useful products at much lower temperatures than current commercial processes. This could lead to processes with substantially lower CAPEX as inexpensive reactor systems can now be utilized. Periana says, “The cost savings realized from not having to use expensive tube and shell reactors and the extensive heat management in current syngas-based processes could be substantial.” This is the objective of the researchers.
Additional information can be found in a recent article (
3) or by contacting Periana at
rperiana@scripps.edu.
REFERENCES
1.
U.S. Energy information Administration, Drilling Productivity Report, March 2014, click
here.
2.
U.S. Energy Information Administration, AE02014 Early Release Overview, click
here.
3.
Hashiguchi, B., Konnick, M., Bischof, S., Gustafson, S., Devarajan, D., Gunsalus, N., Ess, D. and Periana, R. (2014), “Main-Group Compounds Selectively Oxidize Mixtures of Methane, Ethane and Propane to Alcohol Esters,”
Science 343 (6176) pp. 1232-1237.
 Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net.