Cobalt-based thin films
Dr. Neil Canter, Contributing Editor | TLT Tech Beat March 2014
A new study conducted helps to better understand the structure of a self-assembled catalyst found to be effective in splitting water.
KEY CONCEPTS
•
A self-assembled catalyst prepared as a thin membrane film is one of the best catalysts used in the oxygen evolution reaction.
•
A new study has been conducted using x-ray pair distribution analysis to better understand the structure of the self-assembled catalyst.
•
Well-defined and more active nanoscale cobalate clusters were found in the catalyst film prepared from the borate electrolyte as compared to the smaller, less well-defined clusters observed when the catalyst is produced from the phosphate electrolyte.
THE CHALLENGES IN USING SOLAR ENERGY to split water have made it difficult to develop a process that is both effective and durable. One of the reasons is that water can only be split into hydrogen and oxygen at a temperature of 3,000 C, which is not commercially feasible.
Catalyst systems have been looked at to facilitate the process at lower temperatures. In a previous TLT article, research discussed the use of a technique known as isothermal water splitting (
1). The researchers found that a group of cobalt, iron and aluminum oxides can be used to produce hydrogen and oxygen separately in two distinct reactions at a temperature of 1,350 C.
One of the approaches used to split water is known as the oxygen evolution reaction (OER). Simon Billinge, professor in the material science and engineering/applied physics and applied math departments of Columbia University in New York and a physicist at Brookhaven National Laboratory in Upton, N.Y., says, “OER involves splitting water into a hydrogen proton and a hydroxyl anion. The latter then splits into its respective ions, which must find their partners to form hydrogen and oxygen molecules. The two reactions evolving hydrogen and oxygen, respectively, generally require the use of two different catalysts, which may compete with each other, causing problems in efficiently splitting water.”
One of the best catalysts used in OER is prepared as a thin membrane film from an aqueous solution of cobalt oxide in the presence of a buffering electrolyte such as phosphate or borate. The catalyst was developed in the laboratory of professor Daniel Nocera, the Patterson Rockwood Professor of Energy at Harvard University in Cambridge, Mass., with the collaboration of the Billinge group to characterize the structure of the film material.
Billinge says, “The catalyst self-assembles as the film is electrodeposited onto PTO glass plates through a controlled potential electrolysis of the cobalt solution at a pH of either 7.0 or 9.2.”
Remarkably, the catalyst prepared in this fashion does not lose its activity. Billinge says, “In contrast to other catalysts which degrade over time, the self-healing catalyst containing nanoparticles does not become poisoned, even if the environment is dirty. The nano characteristics of the catalyst are needed to provide a high surface area to interact with water molecules, but this work also suggests that the particle size is important for the activity itself.”
Further details about the composition of this self-assembled catalyst would be very useful to better under- stand the structure and then facilitate the optimization of the catalyst. Research has now been done to elucidate the catalyst structure.
X-RAY PAIR DISTRIBUTION FUNCTION ANALYSIS
Billinge and his research associates determined the structure of the self-assembled catalyst through the use of a process known as x-ray pair distribution function (PDF) analysis. He says, “We compared the structures of catalyst films prepared with the phosphate and borate electrolytes in an ex-situ fashion. Measurements were taken on nanoparticles scraped off the membrane film after it was formed.”
One other strategy used by the researchers was to analyze two crystalline structural analogues based on cobalt oxide as a comparison to the membrane films prepared empirically. Billinge says, “Without using the PDF method, it is very difficult to determine structural information from the very tiny signals generated by the nanoparticles, but here structural modeling of the PDF signal, in a quantitative fashion, was crucial to determine thestructure of the membrane films.”
The membrane films produced by the two electrolytes displayed different structures. Billinge says, “We found that the film formed with the borate electrolyte contains a series of well-defined nanoscale cobalt-oxygen (also known as cobalate) clusters that are three to four nanometers in length. A series of three cluster layers form on average with each layer approximately 35 angstroms in diameter.”
Figure 2 shows the catalyst structure for the cobalt oxide film prepared from the borate buffer. Cobalt atoms are depicted as blue spheres, while oxygen atoms are shown as red spheres.
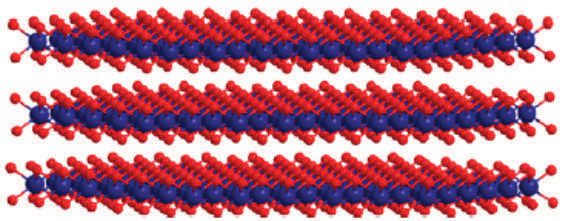 Figure 2. The structure of the cobalate clusters prepared from the borate electrolyte is shown with the cobalt atoms depicted as the blue spheres and the oxygen atoms shown as the red spheres. (Courtesy of Brookhaven National Laboratory)
Figure 2. The structure of the cobalate clusters prepared from the borate electrolyte is shown with the cobalt atoms depicted as the blue spheres and the oxygen atoms shown as the red spheres. (Courtesy of Brookhaven National Laboratory)
Use of the phosphate electrolyte led to the formation of a membrane film that contains smaller cobalate clusters that are not well defined from a structural standpoint. Billinge says, “The clusters formed from the phosphate electrolyte do not yield coherently stacked clusters.”
An evaluation of the catalytic activity of both membrane films shows that the one derived from the borate electrolyte exhibits much better activity. The reason is probably due to the difference in the nano- and meso-scale structures of the two membrane films.
Billinge says, “It appears that a more intermediate order of the nanoclusters leads to a better catalyst. This seems to be counterintuitive because the feeling in many cases is the higher surface area of smaller nanoparticles leads to better catalysis, but the situation is more subtle here.”
The reason for why slightly larger, more ordered nanoclusters provided better performance in splitting water is not known, according to Billinge. He comments, “We do not understand these empirical results. The data suggests that the proximity of the positive and negative charges, so important to facilitate charge transfer, cannot be too close.”
In the case of the nanocluster formed from the phosphate electrolyte, the close proximity may have led to them recombining. Billinge theorizes that there is probably a distance between charges that is optimal. This sweet spot has not been found.
Future work will involve trying to optimize the particle size in the nanocluster in order to find the sweet spot. Additional information can be found in a recent article (
2) or by contacting Billinge at
sb2896@columbia.edu.
REFERENCES
1.
Canter, N. (2013), “Splitting water with solar energy,” TLT,
69 (11), pp. 10-12.
2.
Farrow, C., Bediako, D., Surendranath, Y., Nocera, D. and Billinge, S. (2013), “Intermediate-Range Structure of Self-Assembled Cobalt-Based Oxygen-Evolving Catalyst,”
Journal of the American Chemical Society,
135 (17), pp. 6403-6406.
 Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net.