Use of microwaves to prepare carbon monoxide
Dr. Neil Canter, Contributing Editor | TLT Tech Beat March 2014
Microwaves can be used as a heating source to lower the temperature required to convert carbon dioxide to carbon monoxide.
KEY CONCEPTS
•
A basic process for using carbon to convert carbon dioxide to carbon monoxide is known as the Boudouard reaction and occurs at extremely high temperatures above 700 C.
•
Microwave radiation has now been used in place of convective heating to reduce the temperature of the Boudouard reaction to just above 400 C.
•
One possible mechanism involves the coupling of microwave energy to oxides on the carbon surface, which accelerates the formation of carbon monoxide in the rate-determining step.
FINDING PATHWAYS TO CONVERT CARBON DIOXIDE into a more suitable raw material that can be used to prepare basic fuels such as hydrogen and simple hydrocarbons has been the basis of extensive research. The objective is to develop a cost-effective pathway that artificially simulates photosynthesis.
An example is work underway to facilitate the conversion of carbon dioxide to carbon monoxide. In a previous TLT article, research describing the use of an ionic liquid known as 1-ethyl-3-methylimidazolium tetrafluoroborate (EMIM-BF
4) to convert carbon dioxide to carbon monoxide was discussed (
1). The ionic liquid was found to expedite the formation of carbon dioxide because it formed a complex with an intermediate radical of carbon dioxide.
An even more basic process used to convert carbon dioxide to carbon monoxide is the Boudouard reaction that was discovered in 1905. In this reaction, carbon reacts with carbon dioxide to form carbon monoxide at extremely high temperatures.
Albert Stiegman, professor of chemistry in the chemistry and biochemistry department at Florida State University in Tallahassee, Fla., says, “The Boudouard reaction is used to remove coke from the smelting furnace during the manufacture of steel at temperatures above 1,000 C.”
It is an equilibrium reaction that is extremely endothermic, which means that significant conversion to carbon monoxide only occurs at high temperatures. Better utilization of the Boudouard reaction in commercial applications can only occur if the temperature is reduced.
One potential option is the use of microwave radiation, which we all use to heat up food in our kitchens. Stiegman says, “Microwaves are not suitable for every type of reaction. In many chemical systems, microwaves have no unique effect. One example is comparing the heating of a flask of water in a microwave versus the use of a Bunsen Burner. There is little difference except that microwaves may heat it faster.”
But microwaves can make a difference in reactions where the raw materials are present as a heterogeneous mixture and microwaves can selectively heat a component of that mixture. Stiegman says, “We have found that gas-solid systems represent one of the best chemical systems for using microwaves. In contrast, microwaves have less impact on a fluid solution mixture where the reactants and solvent have similar microwave absorption properties.”
While the use of microwaves may seem foreign, this heating source has been used in the lubricant industry. A U.S. patent application was filed last year for a process that uses microwaves to manufacture greases (
2).
Microwaves have now been used as the heat source to significantly lower the temperature at which the Boudouard reaction takes place efficiently.
SELECTIVE HEATING
Stiegman and his fellow researchers have determined that microwave heating can reduce the activation energy so that carbon monoxide becomes the major product formed at significantly lower temperatures. In particular, a greater than 90 percent conversion to carbon monoxide can be achieved at a temperature of 419 C when using microwaves as compared to 763 C when using convective heat.
The key benefit in using microwaves is selective heating. Stiegman says, “Microwaves have been found to selectively heat carbon, leading to a dramatic change in the thermodynamics of the reaction. As a result, the enthalpy and entropy of the Boudouard reaction decline, pushing the reaction to the right and leading to higher conversions of carbon monoxide at lower temperatures.”
Figure 1 shows the commercial microwave system used to study the reaction. Microwaves at 2.45 GHz were used to penetrate into the carbon.
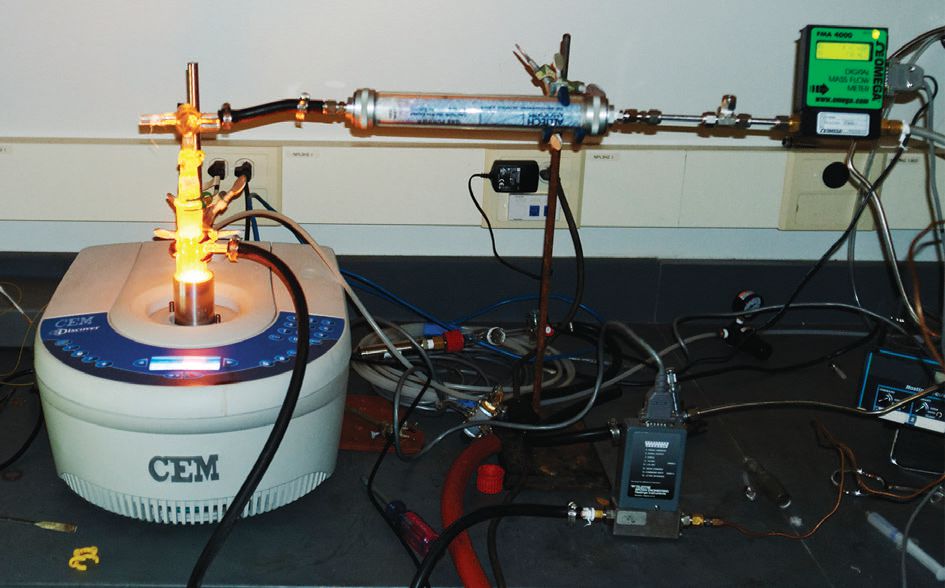 Figure 1. The commercial microwave system used to study the Boudouard reaction is shown. (Courtesy of Florida State University)
Figure 1. The commercial microwave system used to study the Boudouard reaction is shown. (Courtesy of Florida State University)
The researchers studied the process using both flow experiments where carbon dioxide was allowed to move at a constant rate over a carbon sample spread out on a quartz frit in the microwave cavity and using a static experiment. In the latter case, a set volume of carbon dioxide was added by syringe to the microwave cavity containing the carbon. Stiegman says, “We used the static experiments to measure the equilibrium constants for the reaction in the microwave as a function of temperature from which we could obtain the fundamental thermodynamic properties of free energy, enthalpy and entropy.”
Two mechanisms have been proposed for how microwaves expedite the reaction. Stiegman says, “Our main thinking is that microwave energy couples to oxides on the carbon surface and accelerates the ejection of carbon monoxide, which is the rate-determining step for the reaction. A second hypothesis is the incidental microwave radiation creates the formation of transient electron-hole pairs in the carbon. This contributes to the first step of the reaction where in an oxidation process, one oxygen is transferred from carbon dioxide to the surface of the carbon.”
Future work will involve developing optimum reaction conditions for the formation of carbon monoxide. Additional information on the evaluation of the Boudouard reaction using microwaves can be found in a recent paper (
3) or by contacting Stiegman at
stiegman@chem.fsu.edu.
Selective use of microwaves in specific systems appears to have significant potential to accelerate chemical processes. Stiegman, who spends most of his time studying the effect of microwaves on chemical reactions, is now looking at coal gasification. He says, “We are excited to see how microwaves can accelerate the reaction of water with carbon, leading to the formation of synthesis gas (hydrogen + carbon monoxide).”
This work studying coal gasification will hopefully be discussed in a future column.
REFERENCES
1.
Canter, N. (2012), “Artificial photosynthesis: Conversion of carbon dioxide to carbon monoxide,” TLT,
68 (2), pp. 10-11.
2.
Honary, L. and James, W. (2013), “Process and Apparatus for Manufacturing Grease,” U.S. Patent Application Publication 2013/0029887 A1.
3.
Hunt, J., Ferrari, A., Lita, A., Crosswhite, M., Ashley, B. and Stiegman, A. (2013), “Microwave-Specific Enhancement of the Carbon-Carbon Dioxide (Boudouard) Reaction,”
J. Phys. Chem. C.,
117 (51), pp. 26871-26880.
 Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net.