Identification of a rhodium catalyst intermediate
Dr. Neil Canter, Contributing Editor | TLT Tech Beat December 2013
New research may help to improve the compatibility between catalytic converters and automotive lubricants in the future.
KEY CONCEPTS
•
Rhodium is used extensively as a catalyst in chemical reactions including the removal of pollutants produced in an automobile engine.
•
To learn more about how rhodium compounds function as catalysts, a dirhodium-based intermediate has been isolated and characterized.
•
Species similar to the dirhodium intermediate isolated are probably produced on the surface of an automotive catalytic converter.
THE LUBRICANT INDUSTRY is currently under pressure to produce engine oils that meet increasingly stringent regulations yet do not adversely affect catalytic converter performance. Catalytic converters containing noble metals such as palladium, platinum and rhodium remove pollutants, including carbon monoxide, hydrocarbons and nitrogen oxides from the automotive emissions stream. These precious catalysts can be poisoned by lubricant additives such as zinc dialkyldithiophosphates via mechanisms that are not entirely understood. New fundamental insights into the nature of catalytic intermediates in rhodium chemistry can, therefore, be of great importance to the field.
John Berry, professor of chemistry at the University of Wisconsin-Madison in Madison, says, “Rhodium is one of the most important metals used in catalysis. In addition to its use in automotive catalytic converters, important reactions that use rhodium-based catalysts include hydroformylation and hydrogenation.”
One other process that rhodium catalyzes is the insertion of a carbene into a carbon-hydrogen bond. Carbenes are divalent carbon fragments that are extremely reactive. Berry says, “By itself, a carbene is highly reactive and does not discriminate in its reactivity.”
Rhodium interacts with carbenes to form complexes that are much more selective. Berry says, “Dirhodium carbene complexes are able to insert into specific carbon-hydrogen bonds. As an example, this enables simple hydrocarbons to be functionalized in a single step.”
In catalyzing these reactions, researchers believe that the dirhodium complex goes through a series of intermediates. Isolating these highly reactive species has been very difficult, according to Berry. He says, “The speed of these reactions is very fast, and in most of the systems, detection is prevented because formation of the carbene intermediate is the rate-determining step.”
If an intermediate can be studied, then more information can be obtained on the effectiveness of dirhodium complexes as catalysts. A process has now been developed to detect such an intermediate.
RHODIUM-RHODIUM=CARBON FRAMEWORK
Berry and a team of interdisciplinary researchers have identified a dirhodium- based intermediate obtained from the reaction of rhodium carboxylate catalysts with diazo esters. Berry says, “The diazo esters were chosen based on computational studies showing that an intermediate could be stabilized and therefore observed. The diazo esters function as donor-acceptor species where the donor is on one end of the molecule and the acceptor is on the second end of the molecule.”
The researchers faced a major challenge in characterizing the dirhodium-based intermediate. For an organometallic species, the typical approach that would be used is x-ray crystallography. Berry says, “X-ray crystallography is typically used to confirm the structure of a specific substance. Unfortunately, this dirhodium-based intermediate is too reactive to be isolated.”
Instead, the researchers turned to other characterization techniques including ultraviolet-visible spectroscopy,
13C nuclear magnetic resonance (NMR) spectroscopy, mass spectroscopy and x-ray absorption spectroscopy. The first evidence that an intermediate is formed is from a change in the color of the reaction mixture at 0 C. A green-color that is derived from the starting materials is converted to a blue color that fades over time.
Under these conditions, the half-life of the dirhodium-based intermediate is approximately 10 seconds. Berry says, “We determined that the intermediate is based on a rhodiumrhodium= carbon framework in which there is a double bond between the rhodium and the carbon. Evidence supporting this came from the rhodium- carbon interaction in the vibrational analysis, the presence of a specific doublet in the
13C NMR spectrum and confirmation of the molecular weight for the species from the mass spectrum.”
Figure 2 shows an image of the dirhodium-based intermediate where the rhodium atoms are in blue and the carbon atoms are in dark gray. Berry says, “The stability of the dirhodium-based intermediate is based on the solvent used. Deuterated solvents do not readily react with the dirhodium-based intermediates. For the NMR work, the rhodium intermediate is stable in deuterated dichloromethane for about 20 hours.”
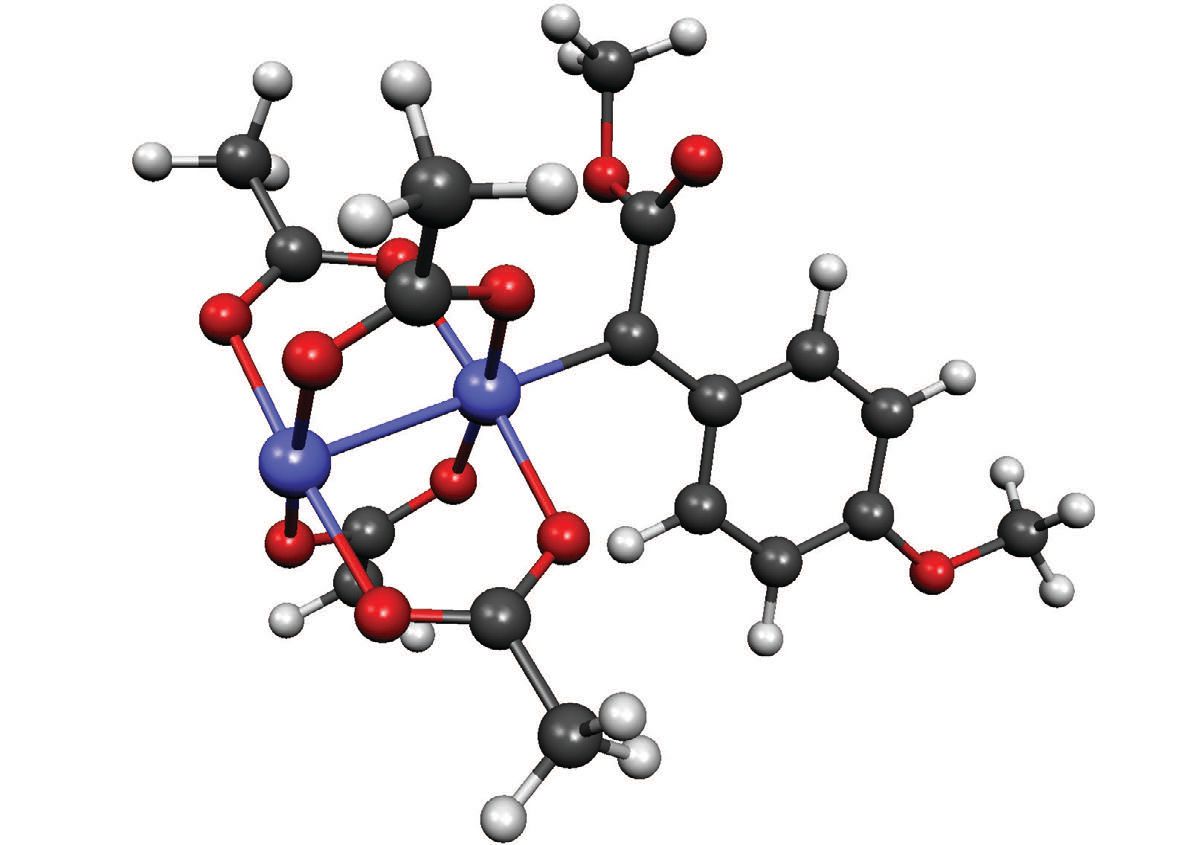 Figure 2. An image of the dirhodium-based intermediate is shown. The key to the reactivity of this species is the metal-metal bond (shown as the link between the two blue rhodium atoms). (Courtesy of the University of Wisconsin-Madison)
Figure 2. An image of the dirhodium-based intermediate is shown. The key to the reactivity of this species is the metal-metal bond (shown as the link between the two blue rhodium atoms). (Courtesy of the University of Wisconsin-Madison)
The rhodium-rhodium=carbon framework is the key to the reactivity of the intermediate. Berry says, “Metal- metal bond is the key to the effectiveness of this noble metal as a catalyst because they are much more reactive than monorhodium species.”
Berry believes that dirhodium intermediates are probably produced on the surface of a catalytic converter, but he is not certain about whether the species identified in this study is involved. He says, “Some type of rhodium- rhodium intermediates are probably formed on the surface of a catalytic converter with carbon, oxygen and nitrogen species.”
Future work will involve two objectives. Berry says, “Now that we can isolate a dirhodium intermediate, we will react it with a variety of compounds to determine what species are produced. We will also continue to look for a dirhodium intermediate that is stable enough to be characterized by x-ray crystallography.”
This research provides insight into rhodium catalysis, which may help in the future to improve the performance of catalytic converters and may also help to improve their compatibility with automotive lubricants. Additional information can be found in a recent article (
1) or by contacting Berry at
berry@chem.wisc.edu.
REFERENCES
1.
Kornecki, K., Briones, J., Boyarskikh, V., Fullilove, F., Autschbach, J., Schrote, K., Lancaster, K., Davies, H. and Berry, J. (2013), “Direct Spectroscopic Characterization of a Transitory Dirhodium Donor-Acceptor Carbene Complex,”
Science,
342 (6156) pp. 351-354.
 Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net.