Hexane isomer filtration using a MOF
Dr. Neil Canter, Contributing Editor | TLT Tech Beat October 2013
An iron-based MOF physically separates dibranched hexane isomers that could lead to upgrading the refining of gasoline.
KEY CONCEPTS
•
Hexane is an important additive used to boost the octane number of gasoline during refining.
•
Past efforts to separate the dibranched hexane isomers which possess the highest research octane numbers are extremely difficult, time-consuming and expensive.
•
A new MOF physically separates the dibranched hexane isomers from the other three hexane isomers, which may lead to upgrading the refining of gasoline.
METAL-ORGANIC FRAMEWORKS (ALSO KNOWN AS MOFs) ARE THREE-DIMENSIONAL CRYSTALLINE STRUCTURES that contain inorganic units joined together by organic linkers. In a previous TLT article, the potential of MOFs was discussed with their original developer, professor Omar Yaghi (
1). He termed the inorganic component as scaffolding and the organic portion as struts.
Yaghi found that a MOF based on octahedral zinc clusters linked by a benzoate derivative shows good performance in adsorbing carbon dioxide. A more recent TLT article highlighted the use of a MOF containing perfluorinated groups (also known as FMOFs) as an oil adsorbent (
2). The FMOFs prepared from silver (I) and a triazolate derivative can adsorb between 190 and 300 kilograms per cubic meters of organic molecules.
High-octane gasoline blends (such as ultra or premium grades used at petrol stations) are prepared through the addition of hexane isomers. Dr. Craig Brown, adjunct professor of chemical engineering at the University of Delaware in Newark, Del., and staff chemist at the NIST Center for Neutron Research in Gaithersburg, Md., says, “Five different isomers of hexane are prepared through a catalytic isomerization process in which each of them are present at concentrations between 10 and 30 percent. The five isomers have different research octane numbers (RONs) with the highest belonging to the dibranched isomers, 2,3-dimethylbutane and 2,2-dimethylbutane.” The values for these two hexane isomers are 105 and 94, respectively.
RONs for the remaining hexane isomers are lower with the monobranched species; 2-methylpentane and 3-methylpentane having significantly lower values (74 and 75, respectively). Finally, the fifth isomer, n-hexane, has the lowest RON which is only 30.
The best approach to using hexane isomers will be to isolate those with high RONs. Brown says, “Unfortunately, all of the hexane isomers are very close in boiling point and extremely difficult to separate.”
Currently, the main process for separating hexane isomers involves using a low temperature, high-pressure process with zeolite 5A that only separates n-hexane from the other isomers. Brown says, “Extremely high pressures of 30 bar and greater and temperatures below 200 K are used in this cryogenic distillation process. The zeolite used has pores with a round shape, which is only useful in separating out n-hexane.”
This expensive and time-consuming process only allows the RON for the hexane mixture to increase to 83. If a method could be established to separate out the high RON, dibranched isomers, then the prospects for upgrading the refining of gasoline significantly improve. An operating example of such a process has now been developed.
IRON-BASED MOF
Brown and his associates at NIST, in collaboration with researchers from several other universities, studied the properties of an iron-based MOF that is known by its chemical structure Fe 2 (BDP) 3 , where BDP is a ligand known as 1,4-benzenedipyrazolate. This MOF has been found to more readily separate the five hexane isomers into the separate dibranched, monobranched and n-hexane components.
Figure 2(A) shows the structure for the precursor ligand, BDP. Figure 2(B) shows an image of the iron-based MOF and Figure 2(C) shows a perpendicular view of the pyrazolate-bridged Fe (III) octahedrea, making up the MOF, which was developed by professor Jeffrey Long at the University of California at Berkeley, who is part of the research team.
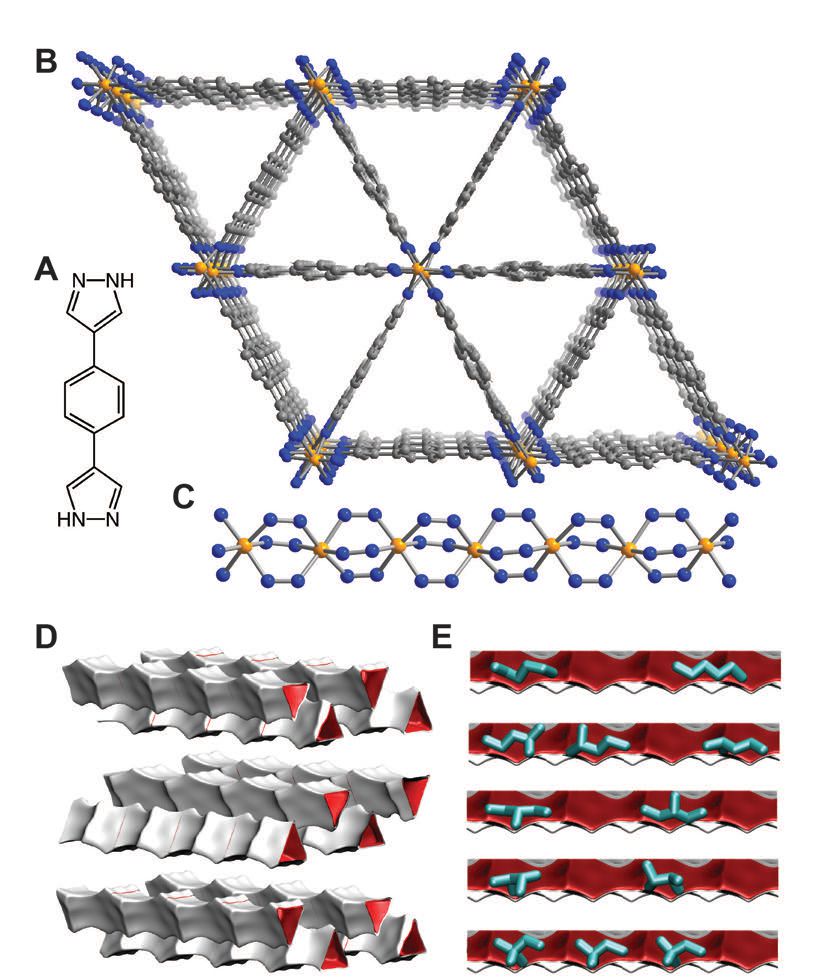 Figure 2. A metal-organic framework developed from the precursor shown in “A” has the triangular geometry seen in “B” that allows for the dibranched hexane isomers to be separated from the other hexane isomers. (Courtesy of NIST and Science/AAAS)
Figure 2. A metal-organic framework developed from the precursor shown in “A” has the triangular geometry seen in “B” that allows for the dibranched hexane isomers to be separated from the other hexane isomers. (Courtesy of NIST and Science/AAAS)
Brown says, “Our studies using neutron powder diffraction show that the MOF’s triangular-geometry is well suited to adsorbing n-hexane, which can be wedged more easily into the pores in comparison to the other branched hexane isomers.” He also noted that one leg of a triangle is 1.3 nanonometers in length.
In contrast to the separation technique currently used, the researchers found that the isomers can be separated at 160 C under a low pressure of 1 bar. Figure 2(D) shows the van der Waals surfaces that are developed within the triangular pores of the MOF. The van der Waals interaction seems to decline as the degree of branching increases, which helps to facilitate separation of the isomers.
In an experiment demonstrating the efficiency of the MOF, a very high RON value greater than 90 was seen in the initial effluent, which consisted mostly of the desired dibranched isomers. The RON declined over the nearly two-hour experiment as first monobranched isomers, and then n-hexane were collected.
The results achieved with the MOF were verified through the use of Monte Carlo (CBMC) simulations. Figure 2(E) shows the presence of hexane isomers in the pores of the MOF that were obtained from these simulations.
The researchers found that the MOF exhibits excellent chemical and thermal stability. This attractive feature, combined with the relatively low-energy intensive process, should make using this MOF commercially attractive to refineries.
Brown says, “The MOF has no chemical functionality at the surface and functions well based on its size and shape. We were very fortunate that its geometry is well suited to separating hexane isomers.”
Future work will involve examining the ability of the MOF to separate chains longer than hexanes. Brown adds, “We also would like to know more about how the individual hexane molecules move through the MOF’s pores, how many of them stick to the surface and how they diffuse through the MOF.”
Additional information can be obtained from a recent reference (
3) or by contacting Brown at
craig.brown@nist.gov.
REFERENCES
1.
Canter, N. (2006), “MOFs: More Effective Gas Adsorbers,” TLT,
62 (4), pp. 12-15.
2.
Canter, N. (2012), “A New Type of Ultra-Stable Oil Adsorbent,” TLT,
68 (3), pp. 14-15.
3.
Herm, Z., Wiers, B., Mason, J., van Baten, J., Hudson, M., Zajdel, P., Brown, C., Masciocchi, N., Krishna. R. and Long, J. (2013), “Separation of Hexane Isomers in a Metal-Organic Framework with Triangular Channels,”
Science 340 (6135), pp. 960-964.
 Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net.