Photographing a chemical reaction
Dr. Neil Canter, Contributing Editor | TLT Tech Beat October 2013
A new technique shows how a molecule is converted into two main products through a well-defined chemical reaction.
KEY CONCEPTS
•
A technique known as noncontact atomic force microscopy has imaged a chemical reaction for the first time.
•
Images of atoms can be seen through the interaction of an oxygen atom at the tip of the atomic force microscope with atoms adsorbed on a flat, silver-single crystal surface.
•
The molecular rearrangement of an oligo-enediyne into two major cyclic products was imaged by this technique.
IN STUDYING CHEMISTRY, reactions are taught showing how atoms are manipulated in specific processes to make new molecules. For those who are chemists, there are a myriad of these processes that have to be learned.
A series of analytical techniques are used such as FT-IR and NMR to show that an actual change in the chemical structure has taken place. One of the frustrations chemists have is the inability to actually see a reaction taking place.
The major emphasis on developing and applying nanotechnology to lubrication has led to the use of new analytical techniques to better understand how atoms interact with each other and to determine the nature of friction and wear at the nanoscale. In a previous TLT article, research was presented that gained insight on the mechanism for wear (
1). A new procedure using an apparatus similar to an atomic force microscope (AFM) placed within a transmission electron microscope (TEM) was used. The researchers evaluated the wear found when a diamond punch was placed in contact with sharp asperities present at the ends of silicon AFM tips. Wear is believed to occur through an atom-by-atom mechanism instead of fracture or plastic deformation.
Dr. Felix Fischer, assistant professor in the department of chemistry at the University of California at Berkeley in Berkeley, Calif., comments on the currently used techniques for characterizing structures at the molecular level. “Methods such as femtosecond laser spectroscopy evaluate molecular transformations by exciting and probing molecules in a molecular beam using pulsed lasers. The problem is that single molecular resolution is not possible and the molecule is vaporized,” Fischer says. “There are also nondestructive subnanometer spatial resolution methods such as scanning transmission microscopy (STM) that provide images of the local density of states rather than the atoms themselves. STM is very effective at imaging the molecular orbitals where electrons are roaming. While this technique works exceptionally well for simple molecules, it can be very challenging to deduce the structure of an unknown, complex molecule using STM.”
Fischer also notes that the structural identification of a molecule using STM is generally aided by a very expensive and time-consuming theoretical modeling process.
A new technique is needed to obtain images of molecules used in specific reactions. Such a method has recently been made available and has been used by Fischer and Dr. Michael Crommie, professor in the department of physics at the University of California at Berkeley, to show how a molecule is converted into two main products through a well-defined chemical reaction.
NONCONTACT ATOMIC FORCE MICROSCOPY
Fischer and Crommie applied a new technique that is known as noncontact atomic force microscopy (nc-AFM) to probe an actual chemical reaction. He says, “Nc-AFM is similar to normal AFM except that it involves the use of a special tip at the end of the cantilever. This tip literally decorated at its apex with a single carbon monoxide molecule that probes the structure of the surface. The apex of this modified tip is thus composed of only a single oxygen atom that interacts with the molecules adsorbed on the surface, leading to much higher resolution imaging.”
The researchers decided to study a reaction known as the Bergman cyclization that was developed by one of Fischer’s colleagues at the University of California at Berkeley, Dr. Robert Bergman. In this process, an oligoenediyne, a molecule that contains three benzene rings linked by alkyne groups, undergoes the cyclization at elevated temperatures to form polycyclic species through a radical cyclization mechanism.
In order to image the reaction, the researchers placed the oligo-enediyne on a clean, flat, silver-single crystal under ultrahigh vacuum conditions to eliminate contamination. Carbon monoxide was then bled into the setup so that it can be picked up by the nc-AFM.
The starting material was imaged by nc-AFM in a process that Fischer indicated takes about 20 minutes. “Even though the instrument used is commercially available, this is not a routine technique. As the AFM tip is moved over the surface, the cantilever vibrates with a very constant frequency,” Fischer says. “When the oxygen atom at the apex of the tip interacts with the atoms of molecules adsorbed on the surface, the frequency of the cantilever changes, indicating the exact position of a single atom or even a bond. Macroscopically, this can be thought of as a form of Braille reading where atoms/bonds are represented as bumps on the surface.”
The change in the vibration frequency of the cantilever is measured by the researchers and leads to the shady pictures generated showing bright and diffuse aspects of the molecules. Figure 1 shows images of the starting oligo-enediyne and the two major cyclic products formed. The researchers found that no reaction occurred until the temperature was increased above 90 C.
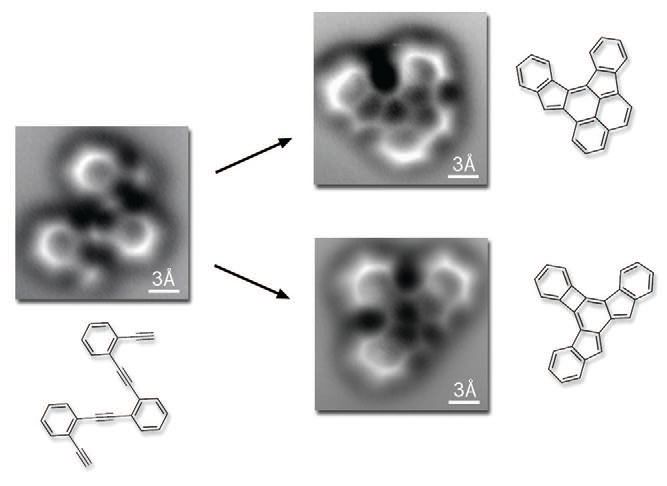 Figure 1. Noncontact atomic force microscopy images show for the first time a chemical reaction that involves the rearrangement of an oligo-enediyne (shown on the left) to two cyclic products (shown on the right). (Courtesy of the University of California at Berkeley)
Figure 1. Noncontact atomic force microscopy images show for the first time a chemical reaction that involves the rearrangement of an oligo-enediyne (shown on the left) to two cyclic products (shown on the right). (Courtesy of the University of California at Berkeley)
From Fischer’s perspective, this reaction was highly atom economic because one molecule rearranged into the products without the addition or subtraction of any other species. He says, “There were no additions or deletions of atoms but, rather, just a rearrangement.”
Of importance for the nc-AFM technique, the molecules are planar. Fischer adds, “We found that trying to study three-dimensional molecules with nc-AFM is still a great challenge because the resolution declines significantly.”
This approach fits in well with Fischer’s objective to take small building blocks on a surface and convert them into higher ordered architecture. He says, “A good analogy is using Legos to make more complicated structures.”
For lubricants, nc-AFM could potentially be very useful as a technique that can actually study how molecules are oriented and provide lubrication on metal surfaces. Further information on this work can be found in a recent article (
2) or by contacting Fischer at
ffischer@berkeley.edu.
REFERENCES
1.
Canter, N. (2013), “Mechanism for Wear at the Atomic Scale,” TLT,
69 (5), pp. 10-11.
2.
De Oteyza, D., Gorman, P., Chen, Y., Wickenburg, S., Riss, A., Mowbray, D., Etking, G., Pedramrazi, Z., Tsai, H., Rubio, A., Crommie, M. and Fischer, F. (2013), “Direct Imaging of Covalent Bond Structure in Single-Molecule Chemical Reactions,”
Science 340 (6139), pp. 1434-1437.
 Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net.