Ironing out the wrinkles in graphene sheets?
Drs. Wilfred T. Tysoe & Nicholas D. Spencer | TLT Cutting Edge October 2013
Chemically modifying graphene changes its frictional properties.
A PREVIOUS CUTTING EDGE ARTICLE discussed the unusual nanotribological properties of atomically thin films of graphite, known as graphene, on surfaces. It was found that the high friction of a single graphene layer, as measured in an atomic force microscope (AFM), could be traced to the formation of wrinkles at the leading edge of the tip, thereby increasing the contact area between the tip and film. These observations suggested that it should be possible to modify the properties of graphene in order to tune its tribological properties. Stiffer films should presumably result in lower friction.
The group of professor Jeong Young Park at the Center for Nanomaterials and Chemical Reactions and the Korea Advanced Institute of Science and Technology, along with collaborators at Konkuk University, took advantage of the ability to chemically functionalize graphene sheets by reaction with fluorine, hydrogen or hydroxyl groups. This changes the number of atoms to which each carbon is bonded, thus modifying the mechanical properties. In pristine graphene, each carbon atom is bonded to three other carbon atoms, resulting in the well-known honeycomb structure. Reaction with another atom leads to carbon atoms being bonded to four other atoms, causing the structure around each carbon atom to become closer to tetrahedral.
Fluorinated graphene was synthesized by reacting a chemical vapor deposition-grown graphene layer with xenon hexafluoride. Subsequent elemental analysis using X-ray photoelectron spectroscopy showed that this resulted in a material with a chemical formula C4F. Functionalization by hydrogen or hydroxide species was achieved by applying a positive or negative bias to an AFM tip in the presence of water vapor. The high electric field between the tip and surface caused the water to dissociate.
Application of a positive bias caused the oxidation of the graphene, while a negative bias produced a narrow strip of hydrogen-modified graphene. The strip is surrounded by pristine graphene, thus allowing the friction of the modified and unmodified graphene to be compared in a single experiment. The resulting structural modifications were confirmed by means of Raman spectroscopy.
The nanoscale friction of the modified and pristine graphene were compared and revealed that the fluorine-modified surface had a friction force about six times higher than that of pure graphene. Oxygen modification caused the friction to increase by a factor of seven, and hydrogen by a factor of two. However, the adhesion between the tip and the modified surfaces was identical to that on pristine graphene.
In order to determine the way in which chemical modification had influenced the mechanical properties, the researchers carried out first-principles quantum calculations using density functional theory. To estimate the in-plane stiffness, they calculated the change in energy of the sheets while changing the spacing between the carbon atoms. The energy was found to vary parabolically with a change in spacing, enabling the force constant to be calculated. A modest reduction in the in-plane force constant by ~35 percent was found.
The out-of-plane stiffness was obtained in a similar way by calculating the energy of the film after moving a carbon atom along a direction perpendicular to the plane. The energy similarly showed a quadratic dependence on displacement. This revealed a substantial effect of chemical modification, with the hydrogenated film being ~1.4 times stiffer than pure graphene, and fluorine increased the out-of-plane stiffness by a factor of ~5.5 and oxidation by a factor of ~7.
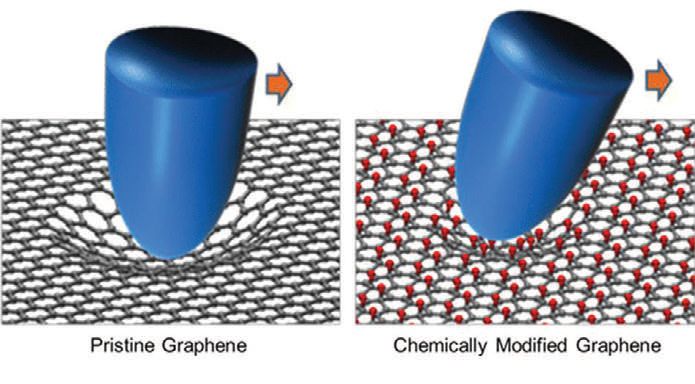
The increase in out-of-plane stiffness correlates very well with the relative friction coefficient; the stiffer the sheets, the higher the friction. However, stiffer films should wrinkle less and it was suggested that bending vibrations could be the main source of frictional energy dissipation.
Clearly, chemical modification of graphene does influence friction, but the cause still remains elusive, suggesting the need for more detailed theoretical studies.
FOR FURTHER READING
1. Tysoe, W.T. and Spencer, N.D. (2011), “A New Wrinkle in Sliding Friction?” TLT, 67(2), p. 72.
2. Ko, J.H., Kwon, S., Byun, I.-K., Choi, J.S., Park, B.H., Kim, Y.-H. and Park, J.Y. (2013), “Nanotrobological Properties of Fluorinated, Hydrogenated and Oxidized Graphene,” Tribology Letters, 50(2) pp. 137-144.

Eddy Tysoe is a Distinguished Professor of Physical Chemistry at the University of Wisconsin-Milwaukee. You can reach him at wtt@uwm.edu.

Nic Spencer is professor of surface science and technology at the ETH Zurich, Switzerland. Both serve as editors-in-chief of STLE-affiliated Tribology Letters journal. You can reach him at nspencer@ethz.ch.